
Caramel sauce, caramel candies, cola, and caramelized onions— these are just a few products of caramelization. But what is caramelization? It may sound like simple, but no, it is not. In fact, like Maillard reaction, caramelization is a very complex process that scientists and experts alike do not fully understand it. But here’s the thing, we’ll discuss things that what science has known so far about caramelization. We’ll answer questions like “why does caramelization create desirable colors and flavors in food?” Or “how to speed up the caramelization process in cooking?”
So let’s dive right in.
WHAT IS CARAMELIZATION?
Caramelization is a process of heating carbohydrates or sugars. The high temperature leads to the formation of new compounds and browning, both of which are desirable changes in food. Caramelization is one of the two forms of non-enzymatic browning that occur in foods, the other is the Maillard reaction. The main difference between the two is that Caramelization involves the pyrolysis of sugars while Maillard reaction is the reaction between sugars and amino acids (proteins).
You might also like: Enzymatic And Non-enzymatic Browning
WHAT EXACTLY HAPPENS DURING CARAMELIZATION?
Caramelization requires a higher temperature in order to occur. Maillard reaction typically starts at a temperature of 212 °F (100 °C) to 284 °F (140 °C). Caramelization, on the other hand, occurs above 248 °F (120 °C) to 356 °F (180 °C). The temperature depends on many variables. And one thing is the caramelization temperature of the sugar. Once the temperature is high enough, sugars, like sucrose, for example, degrade as complex sugar into simple sugars fructose and glucose.
Sucrose is a disaccharide and not a reducing sugar. Hence, must be first converted into simple sugars glucose and fructose.
As heating continues, there is a reduction in water molecules as the simple sugars rejoin to form sucrose anhydride. This is a condensation reaction (also called dehydration) where 2 molecules join in a covalent bond by the loss of a water molecule.
Then, collisions occur that allow for the formation of thousands of volatile compounds, which range from bitter, pungent, to buttery.
While it is possible to heat sugars for an extended period of time, doing so may produce a burned carbon residue. Sugars are made up of rings of atoms of carbon, hydrogen and oxygen. Total oxidation or burning of sugars will convert all the atoms into CO2 and H2O. That leaves nothing behind. This typically happens when the temperature is at 410°F (210°C). This stage of caramelization is also called black jack or monkey’s blood for obvious reason.
Polymer formation
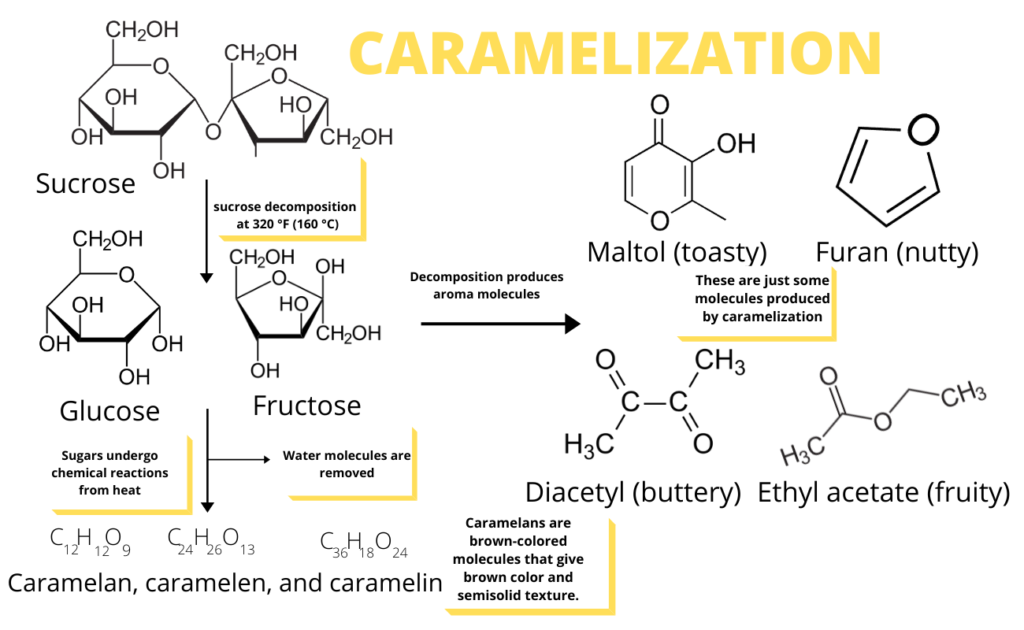
The oxidation leads to long polymers of sugars along with shorter volatile compounds of complex flavors. Polymerization is a process in which small molecules combine to form a polymer.
Commonly, polymers that are made up of up to 6-fused glucose/fructose molecules become chemically modified, producing large, non-volatile caramel products.
Generally, there are three groups of polymers: caramelans (C24H36O18), caramelins (C125H188O80), and caramelens (C36H50O25). They are responsible for the brown colors and flavors during cooking. These large brown molecules are also responsible for the viscosity and stickiness in food.
See below for their distinct characteristics
| POLYMER | CHARACTERISTICS |
| Caramelans (C24H36O18) | First products of polymerization reactions. Form by loss of 12 water molecules. Equals to a weight loss of around 9%. Responsible for the nutty-brown color and bitter taste. |
| Caramelens (C36H50O25) | Darker brown compounds than caramelans. Form by loss of 8 water molecules as sugar condenses with other reactants. Equals to a weight loss of around 15%. |
| Caramelins (C125H188O80) | Products of longer heating of sugar. Darker and deep flavor. Equals to a weight loss of around 22%. Do not dissolve in water very well. |
Formation of volatile compounds

The sweet and buttery, and the creamy sensation that we experience from caramelized foods are products of aroma compounds. These compounds, which include furans like hydroxymethylfurfural (HMF) and hydroxyacetylfuran (HAF) are volatile. Thus, they easily travel into our noses, and bind to our smell receptors.
Furans give off sweet and nutty flavor.
4-methylimidazole (4-MEI), a compound also produced by Maillard reaction, is found in products such as coffee, beers, and some colas as flavor and color.
Diacetyl is responsible for the butterscotch-like and butter taste. It forms in the early part of the caramelization process.
Esters and lactones give rum-like, and also sweet and fruity flavors in food.
Carbocyclic compounds, including cyclopentenone, 3-methyl-2-cyclopentenone give sweet, coffee-like flavor.
To date, there have been over 300 major compounds and several thousand of compounds identified. These numbers just go to show how complex Caramelization is. Nonetheless, studies are in progress to identify more of these compounds, through mass spectrometry. The compounds may be isolated and used as a flavor ingredient in manufacturing.
PRODUCTS OF CARAMELIZATION
Most dishes at the restaurant or at home have caramelization and Maillard reactions happening simultaneously. For example, the golden brown color in bread and other baked products is due to the caramelization of sugars and dextrins to hydroxymethyl furfural and furfural, Maillard reactions, and carbonization of proteins, fats, and sugars. In most cases where there is a large amount of water involved, most of the browning is caramelization.
Whereas Maillard reaction is predominant in low water levels and pH higher than 6. Caramelized onions, baked bread, toffee, toast, and more! It would be hard to imagine how these foods would turn out without caramelization. Vegetables rich in sugars such as corn, potatoes, onions, and carrots are great for caramelization.
In the food manufacturing industry, caramel products are produced as flavoring and coloring ingredients. These may be produced by heating carbohydrates alone, or in the presence of a reactant (base, acid or a salt). Manufacturers of caramel commonly use sucrose, which caramelizes the fastest at at 230°F (110°C). Other sugars such as molasses, invert sugar, D-glucose, and D-fructose may also be used.
One may wonder, why do manufacturers add reactant when producing caramel? Can’t carbohydrates alone be just fine?
Well, as we might observe in many food product labels, caramel has many applications— from baked goods to beers. And these products have their own distinct properties. So plain caramel may just work perfectly for one, but may not be the same case for another. What makes caramel color more ideal is that it does not significantly alter and affect the final product’s flavor profile.
For caramel color in more detail, check this post out: Caramel Color (E150): What Is It As A Food Additive?
FACTORS THAT AFFECT CARAMELIZATION
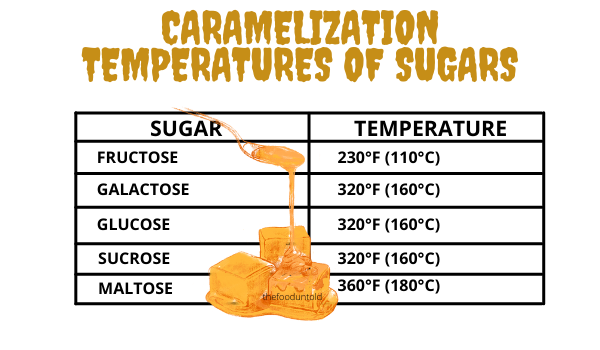
There are several factors that influence the degree of caramelization in food. First things are the type of sugar and temperature. Sugars have varying temperature requirement to enter caramelization. Among sugars, fructose caramelizes the fastest at a temperature of 230 °F (110 °C) because it is more affected by acid degradation. This is why honey, which contains more fructose than other sugars, caramelizes faster than table sugar. Baked products made with fructose or honey have noticeably darker color. Galactose, glucose, and sucrose (table sugar) all caramelize at 320 °F (160 °C). While maltose caramelizes at 360 °F (180 °C).
You might also like: Food Chemistry: The Reasons Why Honey Does Not Spoil
The pH or the acidity of the sugar solution also affects caramelization. At near-neutral pH (around 7.0), the rate of caramelization is slowest. Ideally, you would want to lower the pH to at least 3.0 or raise it to at least 9.0 to accelerate the process. Surprisingly, this still works at lower temperatures! J. Kenji López-Alt of Serious Eats shared that adding a bit of baking soda helps in caramelizing onions much faster. Baking soda or sodium bicarbonate has a pH of around 8.3. Acids like lime juice can also be used to speed up the process.
In producing caramel color, as already discussed, manufacturers use reactants or catalysts to aid in the caramelization process. For example, heating syrup with sulfuric acid (in the presence of ammonia) produces intense-colored polymers (sucre couleur). Aside from sulphite compounds like potassium sulfite (K2SO3), ammonium compounds are also used. One study found out that among catalysts they studied, ammonium hydroxide (NH₄OH) was the better catalyst for caramel production.
Other references:
P. Fellows (2000). Food Processing Technology. Woodhead Publishing
J. Provost, K. Colabroy, B. Kelly, and M. Wallert (2016). The Science of Cooking. John Wiley & Sons. Inc.
J. deMan, J. Finley, W.J. Hurst, and C. Lee (2018). Principles of Food Chemistry (4th Edition). Springer
So that’s about it— the science behind caramelization. Did we miss something? Or you’d like to add something? Either way, leave a comment down below. Much appreciate it.



Pingback:Caramel Buds (History of Australian Candy) - History of Candy
Pingback:4 Ingredient Potato Soup Recipe: Easy, Quick, and Delicious