
Undamaged or uncooked, starch granules are insoluble in water, but can imbibe water reversibly. In the presence of water and heat, starch undergoes gelatinization, a process responsible for the thickening of food systems. The elevated temperature of water allows it to penetrate the starch granules, swelling them until they are fully gelatinized (hydrated). There is also a loss in granular and molecular order within the starch granule. The visible loss in order is characterized by loss of crystallinity and birefringence, and granule swelling.
The swelling of granules results in thickening and the increase in viscosity (resistance to flow) of the liquid. Starches are found in a granular crystalline form, which consists of the polymers amylose and amylopectin. The former assists in gel forming while the latter provides the viscosity.
Starch gelatinization has various applications—most common are in pastas, sauces, and baked products.
In order to manage the texture of foods, they are mostly employed to absorb water and produce viscous fluids, pastes, and gels. The degree of starch gelatinization affects a variety of product characteristics, such as texture, storage stability, and rate of digestion. In pie crusts and low moisture cookies and pie crusts, up to 90% of the starch in the product is not gelatinized. Whereas producing baked products like white bread and angel food cake may include gelatinization of up to 96% of the starch.
Let’s further discuss.
Table of Contents
THE STARCH GRANULE (AMYLOSE AND AMYLOPECTIN)
The starch granule is made up of polysaccharides amylose and amylopectin. Most cereal starches contain 20% to 30% amylose, and 80% to 70% amylopectin. The table below shows the amylose content of common granular cereal starches.
| STARCH | AMYLOSE CONTENT (%) |
| Barley | 22 |
| High-amylose | 52 |
| Maize | 28 |
| Oats | 23-24 |
| Rice | 17-19 |
| Waxy maize | 1 |
| Wheat | 22 |
Both amylose and amylopectin serve as a means of storage of energy (sugar) in plants. The main difference between the two is that amylose is a straight-chain polymer. Several glucose units are arranged in a linear manner through alpha (1-4) glycosidic bonds. Whereas amylopectin is branched polymer, the glucose units are arranged in a branched manner. Because of the branched structure of amylopectin, it exhibits better solubility than amylose.
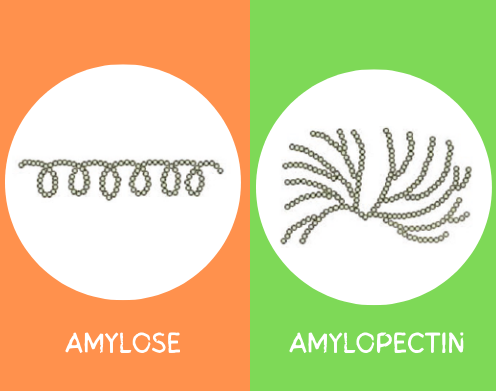
The structure and composition of the starch determine the gelatinization temperature. While the amount of amylopectin branching in the starch affects the gelatinization temperature, a significant amount of amylopectin facilitates the gelatinization process.
You might also like: Why Soaking Potatoes In Water Is Important
Starches lacking amylose thicken but do not gel, whereas those with a high amylose content can gel or keep their shape when molded. The molecules of amylopectin do not form chemical linkages. The more amylopectin that there is, the thicker the starch paste (not a gel), but the more amylase present, the more robust the gel. For more about the difference between amylose and amylopectin, this article should help.
HOW STARCH GELATINIZATION TAKES PLACE
Gelatinization temperature varies depending on the type of starch. (See table below for the gelatinization temperature for each starch). Once the required temperature for gelatinization is reached, the kinetic energy of the heated water molecules breaks the hydrogen bonds between the starch molecules.
Then, starch forms hydrogen bonds with water molecules instead of other present starch molecules. The formation of hydrogen bonds allows water to enter the starch granule further. At this point, swelling of the starch granules take places.As the amylose chains leach out the starch granules, some of them diffuse.
The undercooked granule loses its birefringence and its organized crystalline structure. Due to the expanded granule’s refractive index being so close to water’s, there is a noticeable increase in translucency. Under a hot stage polarizing light microscope, some early stages of gelatinization can be seen. Gelatinization can be measured by observing the decrease of birefringence (the disappearance of the maltese cross). The first stage, the middle, and the last stages are the initial loss of birefringence (initiation).
The range of temperatures at which total gelatinization occurs is established by the complete loss of birefringence.
As the temperature rises, granule swelling increases. The first to swell are the bigger starch granules. Granules that have swollen take up more space and cause the mixture to thicken by releasing amylose and some amylopectin. As the starch paste gelatinizes, it continues to thicken, become more viscous, and flow-resistant.
The final step is cooking the gelatinized starch mix (pie fillings, gravy) for several more minutes for flavor development.

Cooked starches have a variety of features that can be used to further categorize them.Cereal starches (corn, wheat, and rice) form to produce thick, short-bodied pastes that, when cooled, transform into opaque gels. On cooling, the starches in potato and tapioca produce transparent, flimsy gels. High amylose corn starch needs to be heated to high temperatures to gelatinize, resulting in short-bodied, opaque gels that are rigid when cooled.
FACTORS THAT AFFECT GELATINIZATION
Thorough gelatinization of starch is key to producing viscous pastes or strong gels.But to produce high quality gelatinized starch mixture, several factors have to be observed. Here are some:
Temperature
Elevated temperature is required for gelatinization to occur. The completion is up to 203°F (95°C).The temperature varies depending on the starch involved. See below table for the types of starches and their gelatinization temperature.
| STARCH | GELATINIZATION TEMPERATURE (in °C) |
| Common corn starch | 62-80 |
| High amylose corn starch | 66-170 |
| Potato starch | 58-65 |
| Tapioca starch | 52-65 |
| Waxy maize starch | 62-72 |
| Wheat starch | 52-85 |
Gelatinization requires moist heat to take place. The hydrolysis of starch by dry heat results in the formation of shorter chain dextrins (dextrinization). Starch paste viscosity and starch gel strength are impacted by this. Both the paste’s viscosity and the gel’s tensile strength decrease. Flour that has been “browned” by dry heat gives food mixtures a light toasted flavor and a brown tint. Although many recipes could call for this browning result.
Another thing to consider is the length of heating. All granules must have enough time to expand, especially if the granule sizes vary. The resulting mixture might be thinner when the heating time is extended because of the potential for overstirring and rupturing of larger granules. Alternately, cooking for a prolonged period of time in an open double boiler may cause the water to evaporate and thin the mixture.
You might also like: How To Make Stale Bread Soft?
Generally, a starch-water dispersion will typically be thicker at a given endpoint temperature the faster it is cooked.
Acid
The addition of acid typically affects the starch granules negatively during cooking. This results in fragmentation and the production of short chain polymers, such as dextrins. Because less water is absorbed by the starch granule as a result of hydrolysis, the hot paste is thinner and the cooled product is less solid. To simply put, acid hydrolysis forms starch gels that are softer, more elastic, and less cohesive.
However, this can be prevented by adding acid to a starch mixture later, once the starch has gelatinized and started to thicken. Starch sauces are frequently made acidic by adding vinegar, tomatoes, fruit, or citrus juice.
Fat and proteins
One good example of this is meat drippings used for producing meat gravy. Meat drippings is there to initially cover the starch granules’ surface, delaying the hydration and viscosity of the material. The starch granules are “waterproofed” by fat so that water cannot easily pass through them during the gelatinization process.
As a result, there is less granular swelling and less amylose departing from the granule, which lowers the starch paste’s viscosity and weakens the gel’s strength.
This work determined the effect of fat type (beef tallow and poultry fat), the fat content, and the processing conditions on the degree of starch gelatinization of extruded, dry pet food. Results revealed that Increased fat content significantly lowered the degree of starch gelatinization of the extrudates.And in comparison to poultry fat, the addition of beef tallow led to a reduced level of starch gelatinization.
Enzymes
Enzymes are special proteins that act as catalyst of various reactions.
Certain enzymes, including α-amylase, β-amylase, and beta-glucoamylase can hydrolyze starches.
Endoenzymes can breakdown starch anywhere along the starch chain, including undamaged starch granules. Depending on the degree of hydrolysis that occurs, the hydrolysis products of β-amylase are glucose, maltose, and dextrins, and this may be advantageous in the production of commercial bread.
The exoenzyme β-amylase acts on broken amylose or amylopectin chains as well as α-1,4 glycosidic linkages from the nonreducing end. Further hydrolyzing starch two glucose units at a time produces maltose.
Beyond the branch points of amylopectin, β-amylase cannot hydrolyze starch. The 1,4 link in glucose is hydrolyzed by the enzyme -glucoamylase, whereas the 1,6 links in starch are slowly hydrolyzed.
Sugar
Introducing sugar, particularly lactose and sucrose lowers the viscosity of the starch paste, and the firmness of the cooked and cooled starch.
Here’s why.
As we already know, sugar absorbs water as well. For this reason, starch granules will have to compete against sugar and take longer to absorb water. The starch granule won’t quickly or completely swell as a result. Additionally, sugar raises the temperature needed for gelatinization to take place.
If working with both sugar and acid, timing of sugar addition is important. To produce a thicker mixture and gel, it is best to add partially the sugar before the starch thickens and the rest is added later. With this method, there is less sugar competing for water absorption, compared to when all the sugar is added at the start of cooking.
Adding acid and sugar to the starch in the early part of cooking only results in less swelling. First, because of less water absorption and second, less hydrolysis from acid.
Salt
Like sugar, salt is a solute that raises the temperature necessary for gelatinization. But this effect is desirable when cooking pasta. Without proper cooking knowledge, one may end up with a sticky cooked pasta.
You might also like: The Science of Why Salt Is Added To Pasta
Pasta is typically made from wheat starch, which starts to gelatinize at around 131ºF (55ºC). By adding salt, the temperature at which the starch granules increases. In other words, salt decreases starch gelatinization in the pasta. Furthermore, salt reduces water absorption of pasta to keep it firm and not sticky.
Agitation (stirring)
Agitation throughout the starch gelatinization process is necessary to produce a mixture that is uniform—no lumps forming. It allows starch granules to expand apart from one another. However, after gelatinization is complete, vigorous agitation may break starch granules, which may thin starch mixes.
References:
N. A. Michael Eskin, F. Shahidi (2013). Biochemistry of Foods (3rd edition). Academic Press.
S. Damodaran, K. Parkin (2017). Fennema’s Food Chemistry (5th edition). CRC Press.
V. Vaclavik, E. Christian (2014). Essentials of Food Science (4th edition). Springer.
J. deMan, J. Finley, W. Jeffrey Hurst, C. Y. Lee. Principles of Food Chemistry (4th edition). Springer


