
Osmosis is one type of diffusion wherein there is a net movement of molecules that pass through a semipermeable membrane to an area of lower concentration. Examples of semipermeable membrane include synthetic membrane for dialysis and cell membranes in cells of living things like plants and animals. The movement of molecules keeps on until the solute concentration on both sides of the membrane is equal, reaching an equilibrium.
Osmosis is a regular thing in many foods. When fruits are cooked in water, the water migrates into the tissues through osmosis. And sugar, which usually around 12 to 15%, goes through diffusion.
Dried fruits such as raisins become plump. Pectins become soluble and diffuse into the water, resulting in less dense cells and a softer product. Lignins are unaffected by the softening of cellulose. The fruit begins to lose its form.
The main importance of osmosis is its capability to preserve foods and extend their shelf life. Osmosis usually occurs in the presence of salt or sugar solutions. The addition of solutes such as salt or sugar allows the removal of high percentage of water out of bacterial cells to equal the low level of water in the surrounding medium. Sucrose is the most widely utilized osmotic agent in fruits, while sodium chloride is used in vegetables, fish, and meat. Other examples of osmotic agents in food processing include glucose, fructose, lactose, maltose, dextrose, sorbitol, whey, maltodextrin, polysaccharide, and combination of these agents.
This post discusses how osmosis preserves foods and extend their shelf life.
BY LOWERING THE WATER ACTIVITY OF THE FOOD
Just like humans and other living things, microorganisms require water to thrive and multiply. The so-called water activity (Aw) refers to the amount of unbound or free water in the food. This means it is the water available for spoilage microorganisms to consume.
Water activity can be anywhere between 0 to 1.0. Water activity is 1.0 in pure water, whereas the water activity of fresh fruits, vegetables, and meats range from 0.98 to 1.00. This is why they are highly perishable items.
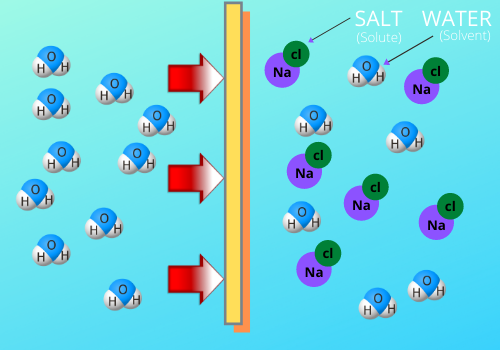
To preserve foods and extend their shelf life against pathogenic bacteria, lowering the water activity via osmotic dehydration is one way to achieve this. Osmotic dehydration is the removal of water from a lower concentration of solute to higher concentration through a semipermeable membrane. This movement of water lowers the water activity and inhibits the growth of spoilage microorganisms.
You might also like: 5 Main Functions of Salt (Sodium Chloride) In Food
Sugar syrup with fruit slices or cubes and salt or brine with vegetables are the most common solutes used in osmotic dehydration. The diffusion process is multicomponent. That means water flows from the fruits or vegetables to the solution, and some of their components, such as minerals, vitamins, and fruit acids, also flow towards the solution. Sugar and salt tend to gravitate toward fruits and vegetables.
This dehydration procedure does not typically result in a shelf-stable product with a low moisture content. As a result, the osmotically treated product should be further processed (usually by air, freeze, or vacuum drying processes) to achieve shelf stability, or the dehydration procedure could be utilized as a pretreatment for canning, freezing, and minimum processing.
Another preservation method that uses osmosis to foods is curing by salt. By lowering the moisture content and serving as a preservative, salting transforms fresh meat into shelf-stable items. These processes, when combined with drying, aid in the formation of distinct sensory properties in the goods, which influence their use as food.
You might also like: Food Science: The Roles of Sugar In Food
BY DIRECTLY ATTACKING THE SPOILAGE MICROORGANISM
The internal osmotic pressure of microbial cells is higher than that of the surrounding medium (food). So the cell wall is subjected to turgor pressure, which provides support the mechanical force required for cell expansion and development. But when the microorganism is in a concentrated aqueous solute solution of reduced water activity because of osmosis, water migrates out of the cytoplasm.
Cytoplasm is the gel-like fluid inside of the cell where reactions take place. The water movement results in loss of membrane turgor. From here, the internal equilibrium (or homeostasis) is disrupted, and the organism will not reproduce.
However, the microorganism will stay in lag-phase until equilibrium is re-established. Lag phase is part of the bacterial growth curve. In the lag phase, microbial population is constant. And the cells are more into adapting to the environment. This post discusses the bacterial growth curve in more detail.
How exactly microbial cells react to osmosis and low water activity?
There are two main ways microbial cells react to adapt to osmosis and changing water activity in food. Here they are.
By accumulating low molecular weight solutes in the cytoplasm
One of the most common responses of cells to lowered water activity is the accumulation of low molecular weight solutes in their cytoplasm at concentrations slightly beyond the external medium’s osmolality. In this manner, the cells regain or prevent water loss through osmosis so the turgor in the cell membrane is maintained for proper functioning.
In 1981, Chirife et al. theoretically calculated the intracellular water activity from the solute content of several bacterial cells cultured in medium with aw values ranging from 0.85 to 0.993. Their study found that the intracellular water activity is equal or somewhat less than that of the growth medium. In terms of cell water content, the overall reaction appeared to represent a homeostatic mechanism.
Salt and sugar are solutes that help preserve food via osmosis. However, not all solutes cause harm to microbial cells.
There are these so-called compatible solutes because they do not interfere with the cell’s metabolic and reproductive functions. What they do is attract water and restore or partially restore isoosmotic conditions across the cell membranes to allow essential metabolic reactions to continue.
They specifically have these properties:
- Small and usually neutral or zwitterionic molecules
- Soluble at high concentrations and can be accumulated in the cytoplasm
- They cell membrane demonstrates permeability to them.
Examples of compatible solutes in bacterial cells include amino acids such as proline, glutamic acid, and amino butyric acid. Predominant protoplasmic solutes in fungi include polyols such as mannitol, arabitol, sorbitol, and glycerol. Many foods that we consume already contain compatible solutes like proline, choline, and betaine to allow growth at low water activity. In some instances, compatible solutes can be delivered from the environment or synthesized de novo in the cell’s cytoplasm.
Adaptation of the membrane composition
Another major reaction of microbial cells to lowered water activity and osmosis is the adaptation of the membrane composition. For many bacterial cells, the most common modification is the increase in the proportion of anionic phospholipids and/or glycolipids in the membrane. Glycolipids serve as receptors for cell-cell communication as well as a structural role in membrane integrity. This adaptation is a way of maintaining the correct bilayer phase to maintain its vital functions.
References:
P. Zeuthen, L. Bogh-Sorensen (2003). Food Preservation Techniques. CRC Press
V. Vaclavik, E. Christian (2014). Essentials of Food Science (4th edition). Springer
M. Shafiur Rahman (2007). Handbook of Food Preservation (2nd edition). CRC Press


