
Whenever we talk about the post-harvest physiology of plant tissues, one of the most discussed are the plant hormones. The so-called classic plant hormones are abscisic acid, auxins, cytokinins, ethylene, and gibberellins. They are plant growth regulators (PGR). Like the name suggests, they aid in the growth and development of cells. Specifically, they help increase return bloom, modify maturity of the fruit, increase branching, and discard excess fruit. Among these 5 growth hormones, studies have been more focused on ethylene, largely because of its direct effect on ripening and senescence, and less is known about the involvement of the other hormones. Furthermore, ethylene’s mode of measurement is relatively easy.
In this article, we’ll discuss ethylene in more detail.
Table of Contents
WHAT IS ETHYLENE?
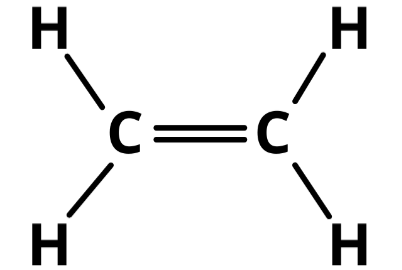
Ethylene (C2H4) is a naturally occurring plant growth hormone in plants. It is a two-carbon hydrocarbon with a double bond (an alkene). It is colorless and nearly odorless (sweet smell of ether). All tissues of the higher plant essentially produce ethylene—from seeds to the fruit.
As a plant growth hormone, it controls many processes in the plant.
And it has a profound effect in these processes, particularly in fruit ripening and senescence, even at trace amounts. Among the 5 PGR, ethylene is unusual. At room temperature, it is a volatile gas. Therefore, it can diffuse quickly between plant tissues or from one commodity to another. This is the reason why bananas, a moderate ethylene producer, help other fruits to ripen faster.
While ethylene has several benefits to plant growth and development, its presence may also be detrimental if not controlled. In fact, a significant portion of post-harvest losses come from hastened senescence and deterioration. One of the common method farmers do to prevent this is harvest the produce unripe, and then artificially ripen it by spraying ethylene. This allows farmers to transport their produce at the optimum freshness and quality.
While delaying the ripening process in fruits minimizes losses, it is good to know which fruits are sensitive to ethylene, and which are not. Fruits picked unripe but have little to no sensitivity to ethylene may never go ripe.
The Food and Drug Administration has approved the use of ethylene as a commercial ripening agent. And no residue standards have been placed, indicating it to be safe.
ETHYLENE SYNTHESIS
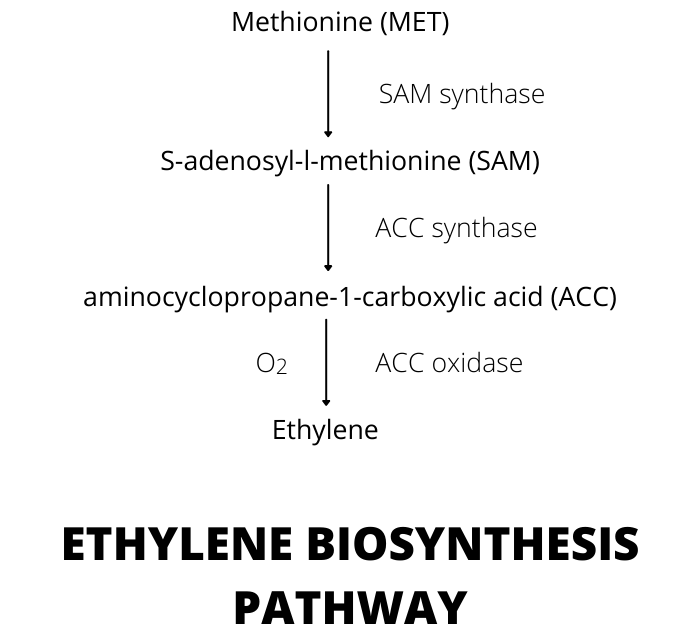
In higher plants, ethylene is produced within plant tissue in a simple two-step biochemical pathway. The synthesis of ethylene involves the amino acid methionine, which is first converted to S-adenosyl-l-methionine (SAM). In the first dedicated step of ethylene biosynthesis, SAM is converted to aminocyclopropane-1-carboxylic acid (ACC). The enzyme involve in this process is ACC synthase, which is normally the rate limiting step. That means an increase in ethylene production is accompanied by an increase in levels of ACC in the plant tissue. The so-called Yang cycle allows the recycling of 5′-methylthioadenosine (MTA), a by-product of this step, to methionine. This leads to the production of ethylene using a small pool of free methionine.
You might also like: Sanitizing Fresh Fruits and Vegetables Using Chlorine
The ACC oxidase (the ethylene-forming enzyme) then converts ACC to ethylene. It is worth noting that the last step of ethylene synthesis requires oxygen. This is why ethylene production is inhibited at low oxygen levels. A study by Hansen (1942) and Burg and Thimann (1959) revealed that ethylene production stopped in apples and pears stored under nitrogen, but production restored upon reexposure to oxygen.
Various studies have also proposed other precursors of ethylene. But it is well established that methionine is the main precursor in higher plants.
CLIMACTERIC AND NON-CLIMACTERIC FRUITS
Based on the rate of respiration, fruits can be classified into two groups: climacteric and non-climacteric. Before we classify fruits, let’s define first what respiration is.
According to The Food and Agriculture Organization of the United (FAO), respiration is basically a reaction of all plant materials. This is the most important physiological activity in plants, and has a direct bearing on the quality of produce. This process involves utilization of sugars produced during photosynthesis and oxygen to produce energy (carbon dioxide, water, and heat) for growth. And it is a continuous process—in the field and after harvest. However, a large number of fruits exhibit a sudden rise in respiratory activity after harvest. This is referred to as the climacteric rise in respiration, a behavior first noted by the upsurge of carbon dioxide gas at the end of the maturation phase of apples. Then after the climacteric, respiration slows down as the fruit ripen.
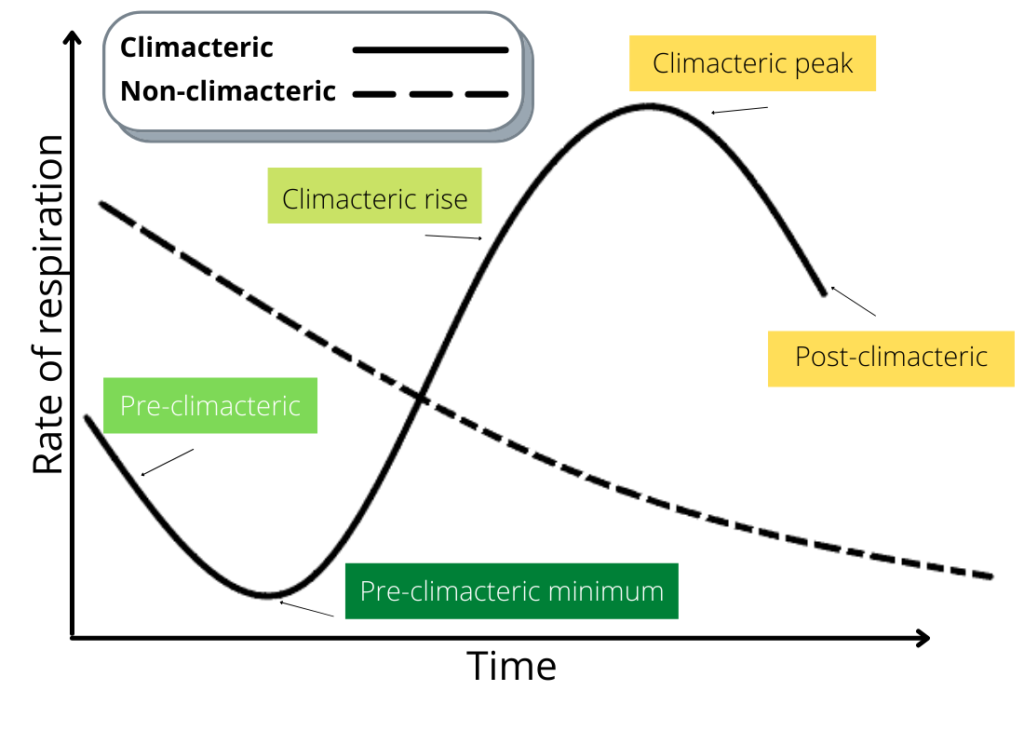
But what does ethylene have to do with respiration in fruits? It is simple. Climacteric fruits produce ethylene as they ripen. This is why climacteric fruits are capable of ripening during the post-harvest period. Non-climacteric fruits, on the other hand, exhibit a steady fall in respiratory activity. Because of this, non-climacteric fruits should only be harvested once they have ripened sufficiently since little to no ethylene is produced once removed from the parent plant. This behavior was first observed in lemons, and then in oranges.
The below table lists some of the climacteric and non-climacteric fruits and vegetables
| CLIMACTERIC | Apple, apricot, avocado, banana, bitter melon, blueberry, breadfruit, cantaloupe, cherimoya, feijoa, kiwifruit, mango, musklemon , nectarine, peach, pear, plum, tomato, watermelon |
| NON-CLIMACTERIC | Blackberry, cacao, cherry, cranberry, cucumber, eggplant, grape, lemon, loquat, mandarin, olive, pepper, pineapple, raspberry, strawberry, summer squash, tamarillo |
ETHYLENE PRODUCTION RATES
Two ethylene systems operate in fruits and vegetables during development and ripening: system I and system II.
You might also like: Are There Fruits That Continue To Ripen After Harvest?
System I ethylene operates in both climacteric and non-climacteric plant tissues as basal ethylene production. It is responsible for ethylene production at immature stages of the fruit. System I ethylene is also responsible for the ethylene production as a result of wounding and other (a)biotic stresses.
At the onset of ripening, system II ethylene takes over. At this point, the production of ethylene switches from autoinhibitory to autostimulatory. In climacteric fruits, the transition is characterized by the sudden increase in autocatalytic ethylene production, accompanied by a spike in the rate of respiration. During ripening, ethylene production is associated with the upregulation of the enzymes ACC synthase and ACC oxidase, both of which increase in activity.
We have listed some climacteric fruits (and vegetables). But they are not created equal. Which ones produce ethylene more? The distinction between climacteric and climacteric fruits was first based on the pattern of respiration. Today, they are identified by the differences in ethylene production.
The below table shows the classification of fruits and vegetables according to ethylene production rates.
| CLASSIFICATION | PRODUCTION RATE (μL C2H4/kg · h) at 68 °F (20 °C) | FRUIT/VEGETABLE |
| Very low | <0.1 | Artichoke, cauliflower, potato cherry, asparagus, citrus fruits, green vegetables, grape, jujube, pomegranate, root vegetables, strawberry |
| Low | 0.1-1.0 | Blackberry, blueberry, casaba melon, tamarillo, cranberry, olive, eggplant, raspberry, okra, cucumber, pepper, persimmon, pineapple, pumpkin, watermelon |
| Moderate | 1.0-10.0 | Banana, tomato fig, guava, honeydew melon, lychee, mango, plantain |
| High | 10.0-100.0 | Apple, plum, cantaloupe apricot, pear, avocado, nectarine, feijoa, kiwifruit, papaya, peach |
| Very high | >100.0 | Passion fruit, cherimoya, sapote, mammee apple |
METHODS OF CONTROLLING ETHYLENE
Ethylene is generally beneficial. It helps the firmness of the fruit to soften, increase its sugar content, release aroma compounds, and develop flavor. But if uncontrolled, may result in post-harvest losses (hastened senescence, and deterioration). This is especially true for produce meant for long-term storage or transport to distant places. In some fruits and vegetables, ethylene may induce certain undesirable effects. These include yellowing in brocolli, sprouting in potato, and color loss in leafy vegetables.
To delay or inhibit the action of ethylene in fruits and vegetables, several methods can be used.
Low temperature

In general, low temperatures slow down metabolism, and this include ethylene production in plant tissues. This is the reason why it is ideal to store the produce at chilling temperature. But when storing, it is good to know the compatibility of the produce in relation to tolerance to ethylene. Produce that does not tolerate ethylene must be stored away from another that generates ethylene at a fast rate.
Another reason to separate produce during storage is that some tropical fruits are chilling-sensitive. For most temperate produce, the optimum storage temperature is 30–32 °F (-1.0-0.0 °C) for the non-chilling-sensitive varieties, and 38–40 °F (3.3-4.4°C) for the chilling-sensitive varieties. For more on susceptibility of fruits and vegetables to chilling injury, visit this guide by FAO.
You might also like: The Reason Why Sliced Apples Turn Brown
Oxygen removal
Oxygen is essential in ethylene production. Hence, an environment where the atmosphere is controlled helps delay ripening. This study investigated the effect of ethylene, oxygen, and carbon dioxide on the ripening process of bananas. Results revealed that high CO2 concentrations and low O2 concentrations retarded ethylene synthesis as well as ripening in the uninitiated fruit.
A similar study investigated the effect of low O2 and high CO2 on ethylene and ACC in ripening apples. Results revealed low ethylene production at the climacteric stage in the presence of either high CO2 and low O2. However, low O2 only inhibited the conversion of ACC to ethylene. Whereas high CO2 inhibited the formation of ACC, as well as the conversion of it to ethylene.
Modified atmosphere packaging is one good example of inhibiting ethylene production by oxygen removal. In this method, the packaging material is enriched with CO2 and deprived of O2.
Use of ethylene biosynthesis inhibitors
Ethylene biosynthesis inhibitors, like the name suggests, help delay ripening in fruits and vegetables by interrupting the ethylene biosynthesis. In studying the action of ethylene in plant tissues, three inhibitors are commonly utilized:
- 2-aminoethoxyvinyl glycine (AVG)
- Silver ions (Ag)
- 1-methylcyclopropene (1-MCP)
AVG is an inhibitor of ACC synthase while both Ag and 1-MCP are inhibitors of ethylene receptors. Studies have revealed AVG to be an effective ACC synthase (the key enzyme in ethylene biosynthesis) inhibitor. It can be applied as a pre- and post-harvest treatment to delay ripening. One study applied pre-harvest spray of AVG prior to the commercial harvest of several varieties of apples, including Senshu, Red Fuji, Redchief Delicious, and Lodi. The pre-harvest treatment resulted in higher firmness and reduced respiration for 30 days.
Silver ions help retard ripening by competing for the binding sites of the receptors of ethylene. However, they are more potent if applied as silver thiosulfate (STS). It is more mobile and less toxic than silver nitrate.
1-MCP works by also by binding to the ethylene receptors. Aside from delaying ripening in fruits, 1-MCP also works to maintain the freshness of flowers and ornamental plants. A gaseous compound, 1-MCP is often used in enclosed areas such as greenhouses and storage facilities.
Other references
deMan, J. Finley, W. Jeffrey Hurst, Chang Yong Lee (2018). Principles of Food Chemistry (4th edition). Springer.
S. Damodaran, K. Parkin (2017). Fennema’s Food Chemistry (5th edition). CRC Press.
H. Ramaswamy (2015). Post-harvest Technologies of Fruits & Vegetables. DEStech Publications, Inc.
N. A. Michael Eskin and F. Shahidi (2013). Biochemistry of Foods (3rd edition). Elvesier Inc.
P. Golob, G. Farrell, and J. Orchard (2002). Crop Post-Harvest: Science and Technology (Vol. 1 Principles and Practice). Blackwell Science Ltd.


