
Freeze drying is one of those food processing methods that involves the removal of heat. The modern way of freeze drying started in the late 1800s when Richard Altman developed a method of freeze drying of tissues of animals and plants. Unfortunately, the public never took notice of his work for over 40 years. But in 1932, the first vacuum freeze drying plant was built. And the advent of the Second World War helped the process of freeze drying to be of great importance. During the war, refrigeration of medicines (especially plasma and penicillin) and food was uncertain. This is especially true when they needed to be transported to distant war zones. However, the-then novel method of preservation helped them become stable and more resistant to spoiling. For this reason, the process of freeze drying became an industrial process.
You might also like: How Instant Coffee Is Made (And What Is Lost From Ground Coffee)
After the war, the NASA Space program also turned its attention to the process when they needed a method to keep foods from spoiling while retaining their nutrients. In fact, the astronauts aboard the Gemini in 1965 brought some freeze dried food items in space. That same year, multinational company, Nestlé, released in the market Nescafé Gold Blend, the first ever freeze dried coffee.
Today, freeze drying is used for a wide range of foods, including juice, vegetables, eggs, dairy, coffee, and food flavorings. Often times, freeze-dried foods are packed in moisture-free, oxygen-absorbing materials. And when properly stored, the product can have a shelf life of more than 12 months.
You might also like: What Is Chilling in the Food Industry?
In this post, we’ll define what freeze drying is and how it works.
WHAT IS FREEZE DRYING?
Freeze drying is one form of food preservation by drying. However, freeze drying uses low temperature to dehydrate the product and preserve it. This way, the product is frozen, and subsequently vacuumed (between 27–133 Pa) to evaporate the moisture in the process of sublimation. Sublimation is the conversion of ice directly from the solid state to the gas state (vapor) without passing through the liquid state.
Freeze drying is ideally used only for food items that can be sold at a high price, like coffee. Freeze drying is slower than conventional dehydration, evaporation or membrane concentration. Aside from the energy costs, the production of a high vacuum is an additional expense. This, together with a relatively high capital investment, results in high production costs for freeze-dried foods. The cost of freeze drying is up to four times more than conventional drying.
For this reason, freeze drying is a more important operation commercially for expensive dry foods which have delicate aromas or textures. This is the reason why expensive freeze dried foods such as coffee, herbs, spices, mushrooms, meats, seafoods, and vegetables are superior in quality. Volatile aroma compounds are entrapped in the food matrix rather than being entrained in the water vapor created by sublimation. As a result, 80–100% aroma retention is feasible.
There are also minimal effects on the nutritional properties of foods. Although freeze drying is a form of drying, the temperature is below the freezing point. Therefore, its preservative effect is attained by reduction of water activity without heating the food. Hence, the nutritional properties are as well retained. This is very opposite of that of conventional drying, where the temperature is between 98.6°F (37°C) to 199.4°F (93°C). This is the reason why freeze drying is believed to be the best form of drying food.
HOW IT WORKS
Freeze drying starts by freezing food using conventional freezing equipment. This is the pre-freezing stage. To reduce the damage to the cell membrane of the food, small pieces of food is quickly frozen to allow small ice crystals to form. Large crystals will only break the cell walls of certain food which may result in loss of texture and nutrients. However, Slow freezing is beneficial in liquid foods since it creates an ice crystal lattice, which creates channels for the flow of water vapor.
The next step is the removal of the ice through sublimation. This is the primary drying stage. At this stage, temperature and pressure have to be carefully monitored as the rate of sublimation depends on these two. The difference between the vapor pressure of the food and that of the ice collector specifically makes the difference. The vapor pressure is the rate of how fast a liquid evaporates. As the temperature increases, the vapor pressure increases as well. Molecules tend to move from a higher pressure to another of lower pressure. For this reason, the temperature of the food should be higher than that of the ice collector.
After drying, all of the ice has already been removed via sublimation. At this stage, the moisture content of the food is at approximately 15%. The unfrozen water present is removed by evaporative drying (isothermal desorption) until the moisture content decreases to 2%. This is the secondary drying. Desorption, the released of absorbed substance (water), from the surface, is done by raising the dryer temperature to near ambient temperature while retaining the low pressure.
The rate of drying depends on two factors: the resistance of the food to heat transfer and on the resistances to vapor flow from the sublimination front.
FREEZE DRYING EQUIPMENT
Freeze dryers are distinguished by the method of how heat is applied to the surface of the food. Radiation and conduction types are common industrially. Another type is microwave freeze drying. These types of dryers are available in both batch and continuous versions.
Freeze dryers consist of 4 basic parts: a vacuum chamber, a vacuum pump, a heat source, and a condenser. The vacuum chamber contains trays to hold food during drying, as well as heaters as a source of latent heat of sublimation, or the heat required to change the state of a substance from solid to gas, at a constant temperature. As the name suggests, drying occurs in a vacuum to prevent oxidation from occurring. Refrigeration coils are utilized to condense the vapor straight to ice.
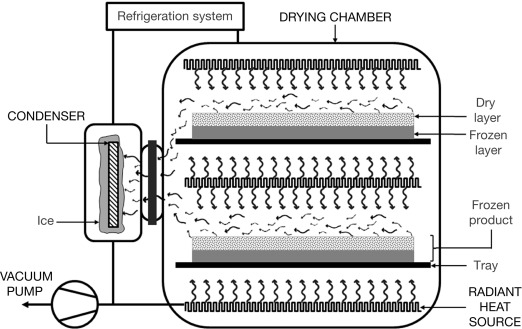
These coils come with automatic defrosting devices to control the formation of ice for vapor condensation.
Between the chamber and the condenser is a vacuum pump, whose function is to remove non-condensable vapors and reach the ideal vacuum level of below 0.61 kPa (4.58 mm Hg). It comes with an inlet for steam. Without a vacuum pump, the freeze dryer will not function.
Once the frozen product reaches the target high vacuum level, the heat source provides the latent heat of sublimation. The temperature of the heat source varies between 243.15 (−30°C) and 423.15 K (150°C).
The condenser collects water vapors produced by the sublimation of ice inside the product. Around it is a mixture of dry ice and acetone to maintain a temperature lower than the frozen material. When water vapor comes into touch with the condenser surface, it transforms into ice crystals that release energy before being evacuated from the system.
Other references
V. Vaclavik, E. Christian (2014). Essentials of Food Science (4th edition). Springer.
P. Fellows (2000). Food Processing Technology (2nd edition). Woodhead Publishing Limited.


