
I live in a tropical country. So it makes perfect sense that ice cream is an everyday food for us, especially for children. A common scenario here is an ice cream vendor peddling his product on a hot afternoon. When I was younger, I used to wonder why they immerse ice cream tubs in ice and salt bath before they head out. But I never got the chance to ask them why. Does it enhance the flavor? Does it make the ice cream more resistant to melting? It turned out that ice cream makers have been using salt and ice bath in making their product since the early 19th century. They do this to actually make ice cream freeze faster. But how does it work?
You might also like: Emulsifiers In Food: What Are They?
Because as far as I know, countries away from the equator use salt to melt ice. When it snows, people would spread salt on roadways to prevent the snow from freezing, keeping the road from being icy and dangerous for travelers. This works because salt messes with the freezing point of water, impeding the ability of water to form ice crystals.
But how does salt and ice bath work the other way in making ice cream?
SALT AND ICE LOWER THE FREEZING POINT
Under normal conditions, pure water freezes at 32°F (0°C). But this temperature is not sufficient to freeze the liquid ice cream. Milk is one of the main ingredients in making ice cream. It starts to freeze at 31.1°F (0.5°C). A typical ice cream would also include ingredients such as sugar, color, and flavor. For this reason, a temperature colder than ice is necessary to freeze the ice cream. Generally, ice cream freezes at -15°F (5°C) or lower. But with enough salt (sodium chloride) and ice, temperatures of -4°F (-20°C) can be reached, helping the ice cream to freeze faster.

The combination of salt and ice do not melt the salt though. Instead, these two lower or depress the melting point of the ice water—a colligative property. In chemistry, a colligative property is the property or characteristic of a substance that varies according to the number of particles of solute (salt in this case) in a solvent (ice or water).
This does not depend on the chemistry or the nature of the particles, just the number of particles. The more particles dissolved, the greater the impact on freezing point depression.
FREEZING POINT DEPRESSION
When we take ice out of the freezer, its temperature is roughly the same as the inside of the freezer. But as it stays out, it starts absorbing heat, raising its temperature. We all know that water freezes at -32°F (0°C). Once the ice reaches this temperature, it begins to melt. But in the presence of salt, its melting point can be lower than 32°F. Hence, a salt and ice water bath can stay liquid even at a temperature below 32°F—this phenomenon called freezing point depression can help freeze our ice cream before the ice melts completely.
You might also like: The Science Of Making Ice Cream
Salts are ionic molecules—the bonds have electric charges. The chloride ions are negatively charged, whereas the sodium ion is positively charged. These are opposite charges that attract one another. However, the ionic bond breaks in water because the ions is solvated by water molecules. Sodium chloride dissolves into sodium cation and chloride anion. For each molecule of NaCl in water, the sodium and chloride particles are able to impact the freezing point depression.
The freezing point depression can be measured by the below formula.
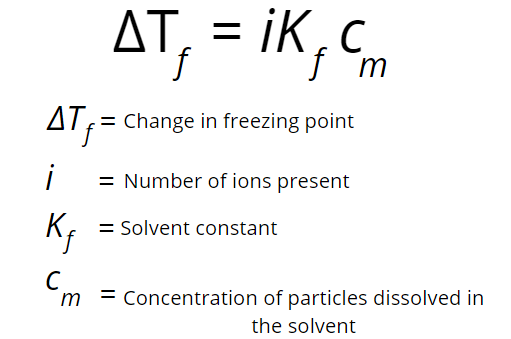
Based on the formula above, we can see that more particles means a higher total concentration and more effect in the freezing point. But only impurities such as salt have the capability to do this. When dissolved in water, sugars such as lactose and sucrose do not dissociate into ions. Therefore, one molecule of NaCl has twice the effect on the freezing point than does sucrose.
MAKING ICE CREAM AT HOME
Want to see how freezing point depression works at home? Sure, you can by freezing ice cream without using your home freezer! You will only need three components here: fat, water, and sugar. So ready your whipping cream (1/2 cup), sugar (1/4 cup), milk (1/2 cup), and some vanilla (1/4 teaspoon) for flavor. Place these ingredients in a mixing bowl and mix them until the sugar has dissolved. After that, pour the liquid ice cream in a medium sealable bag. Make sure to squeeze out all the air before sealing the bag. In a larger sealable bag, place crushed ice. Try to measure the temperature of the larger sealable bag. if you have a thermometer. You should get a reading of around 24.8°F (-4.0°C).
You might also like: UHT And Pasteurized Milk: What’s The Difference?
Now add one cup of salt in the sealable bag. Bigger coarse salt crystals work better. Wait a little moment and then read the temperature again. You should get a reading much lower than that with crushed ice alone. With calcium chloride hexahydrate salt, a calcium salt, temperatures of around −40°C can be reached.
Place the sealable bag with liquid ice cream inside the bag filled with ice and salt. Now shake the bags for 10 to 15 minutes. What happens here is that the ice starts to melt as it absorbs heat from the ice cream mixture. In return, the temperature of the ice cream becomes lower.
After shaking, check the bag if the ice cream is already solid. Otherwise, refill the larger bag with crushed ice and shake the bags again for several more minutes. Remove the inner bag and enjoy your ice cream.
So that is how salt helps freeze ice cream. Want to learn more about freezing point depression? The Chemistry Library discusses freezing point depression in more detail.


