
Tartaric acid is a weak acid naturally present in grapes, pineapples, and bananas. Tartaric acid is also the major acid in wine and wine vinegars. For this reason, it is an excellent marker for grape wine as only a few other fruits produce the acid in significant quantities. Furthermore, only a few microbes are capable of metabolizing tartaric acid. Tartaric acid has various functions in food. This acid is specifically preferred when increasing the acidity level of high pH wines.
Tartaric acid comes in the form of salts, which include calcium tartrate, potassium tartrate, and sodium tartrate. Winemakers has been incorporating tartaric acid in their products. Although the first extraction method was developed only in 1769 by Carl Wilhelm Scheele, a Swedish German chemist. Scheele was also the first to isolate oxalic, lactic, citric, malic, mucic, prussic, and uric acid.
Today, tartaric acid is one of the most commonly used organic acid in food manufacturing. It is often used as an acidulant, due largely to its Low pKa values (3.03 and 4.54). In comparison to citric acid, tartaric acid has a smoother tartness profile and a greater peak acid taste that is also long lasting. Among the regularly used acids, tartaric acid may be the most expensive.
Let’s discuss further.
Table of Contents
WHAT IS TARTARIC ACID
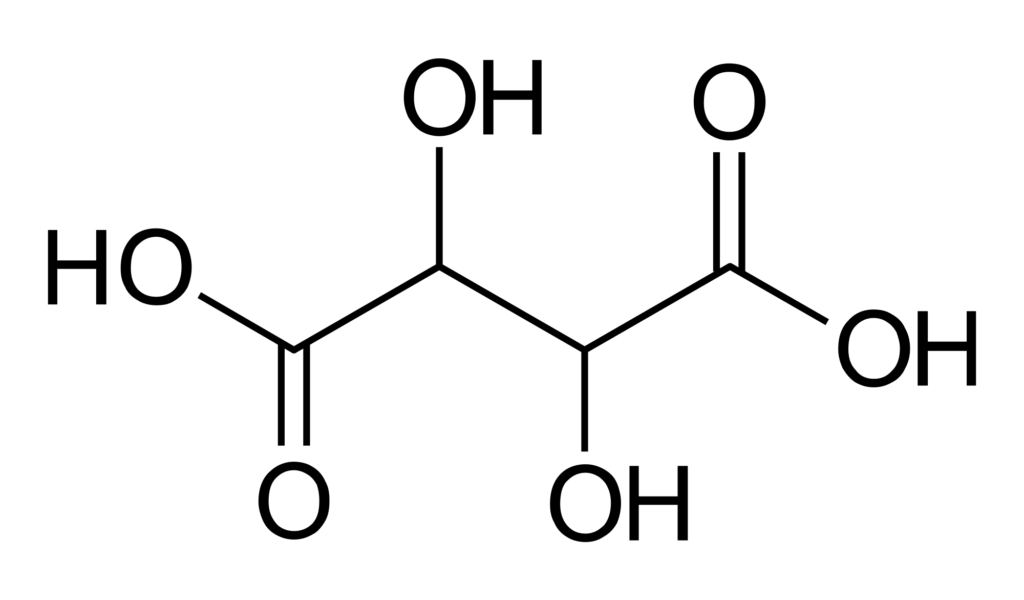
Tartaric acid is one of the hydroxy acids (others include citric acid, glycolic acid, oxalic acid). It appears as a white, odorless crystalline powder. It is readily soluble in water at 1.33 kg/L. For more of its properties, visit PubChem.
Its chemical formula is C4H6O6.Two acid groups and two alcohol groups are present.
The molecule’s second and third carbons are asymmetrical. The L-isomer of tartaric acid, which spins slightly to the left, is the form that occurs naturally. The acid’s D-form, which rotates plane-polarized light in a right-hand direction, is much less frequent in nature.
Malic acid and tartaric acid comprise 70% to 90% of total grape acidity.
As a food additive, tartaric acid functions as an acidulant, flavoring agent, leavening agent, chelating agent, and to a lesser extent, as a preservative. It is the major acid in wine. One of its derivatives, potassium bitartrate or cream of tartar is a byproduct of winemaking. It is responsible for the so-called “wine-diamonds” crystals that form on the bottom or cork of the wine bottle. Tartaric acid is also present in considerable amounts in wine vinegar and balsamic vinegar.
The E number of tartaric acid is E330. E numbers represent as code for the food additives used in the European Union.
PRODUCTION METHODS
Tartaric acid can be produced by natural or synthetic methods.

Industrially, the L-(+) -tartaric acid isomer or dextro-tartaric acid (naturally occurring form) of tartaric acid is available in high amounts. This form of tartaric acid is cheaper than meso isomer. This is produced by obtaining leeds, which are deposits from left over yeasts and other particles that precipitate to the bottom after fermentation and aging of wine. This is one of the reasons why tartaric acid is more expensive than most acidulants.
Chemically, leeds are composed of potassium bitartrate (KHC4H4O6). This is treated with calcium hydroxide and converted into calcium tartrate (CaC4H4O6).
To efficiently produce more calcium tartrate, calcium chloride (CaCl2) is added. By treating the salt with aqueous sulfuric acid, tartaric acid is produced, which is separated, purified so it is in its commercial form.
The more expensive meso-tartaric acid can be produced from dextro-tartaric acid. Production starts by heating dextro-tartaric acid at 329°F (165°C) for 48 hours. This produces racemic acid and meso-tartaric acid, which is then separated from the mixture.
This Google patent describes a method of producing tartaric acid, which 55% to 90% is meso-tartaric acid.
FOOD APPLICATIONS OF TARTARIC ACID
Tartaric acid and its salts have various uses in the food industry. They work as a:
- Acidulant
- Chelating agent
- Flavor enhancer
- pH control agent
- Stabilizer
It is worth noting that tartaric acid is the least antimicrobial among organic acids (acetic, ascorbic, benzoic, citric, formic, fumaric, lactic, levulinic, malic, and propionic acids). Although it can lower the pH, it can inactivate only a fewer number of microorganisms.
As Acidulant/flavor enhancer
Like other organic acids, tartaric acid gives the tart, sour taste of fruits. As already mentioned earlier, tartaric acid is the dominant acid present in wine grapes. Being an acidulant is the most often role of tartaric acid in many food products, including wines, juices, sodas, jams, jellies, marmalades, and confectionery.
You might also like: Food Preservative: What Is Sorbic Acid (E200)?
The concentration of it in the fruit as well as that added during vinification are directly related to the organoleptic and aging potential of wines.
However, titratable acidity is not a measure of tartaric acid in wine. The tartaric acid equivalent is the unit used to measure wine acidity. A wine can contain 0 g/L tartaric acid, but still contains 5 g/L “as tartaric acid”.
In aged wine, tartaric acid and oxygen combine to form glyoxylic acid in the presence of Cu2+ or Fe2+. This new substance binds phenolics, such as tannins, producing a more developed, less astringent flavor.
As Chelating agent/stabilizer
Chelating agents or sequestrants are important for food stabilization because they react with metallic and alkaline earth ions to generate complexes that change the ions’ characteristics and how they affect food. Numerous metals are naturally chelated. These include copper, zinc, and magnesium. They take part in producing undesirable changes in food such as oxidative rancidity flavor or color change. The majority of chelating agents used in the food industry are natural substances, which include polycarboxylic acid like tartaric acid. The addition of tartaric acid form complexes with these metal ions to stabilize the food.
As pH control agent
Cultured milk products are produced by fermentation through the addition of bacterial culture, such as Lactobacilli and Streptococci. During the process, microorganisms convert lactose into lactic acid, lowering the pH in the process. In some dairy products, tartaric acid is added, with or without microorganisms, to acidify them.
Invert sugar is a syrup mixture of 50% fructose and 50% glucose. Its advantage over regular sugar is its better moisture retention, and increased solubility. For this reason, it is ideal for making candies. One way to produce invert sugar is by hydrolyzing sucrose by a weak acid, usually cream of tartar. The presence of cream of tartar produces equal amounts of glucose and fructose. The amount of the acid must be carefully observed as too much of it will result in excessive hydrolysis. This will only produce a soft or runny sugar product.
As leavening agent
In baking, baking soda is oftentimes partnered with cream of tartar. Baking soda is an alkaline, whereas the cream of tartar is an acid. The reaction between the alkaline and acid produces carbon dioxide or bubbles within the dough or cake to leaven it. Baking powder is similar to baking soda. However, it is already a mixture of baking soda, cornstarch and cream of tartar. The production of carbon dioxide starts once baking soda comes into contact with cream of tartar once the baking powder becomes wet.
You might also like: The Science Of Rising Bread (And Why Yours Is Not)
HEALTH CONCERNS
Tartaric acid is generally regarded as a safe ingredient in foods. Since the human body does not metabolize tartaric acid, it can be consumed without side effects.
According to the Food and Drug Administration (FDA), tartaric acid meets the specifications of the Food Chemicals Codex, 3d Ed. (1981), P. 320. And that it is generally recognized as safe (GRAS) as a direct human food ingredient.
In the European Union, the Scientific Committee for Food (SCF) established the acceptable daily intake (ADI) of 30 mg/kg body weight per day, for tartaric acid and its salts.
Numerous toxicological studies on tartaric acid revealed no toxic effects, including nephrotoxicity. Monosodium l(+) tartrate was studied over a long period of time in rats, and the greatest dose examined (3,100 mg/kg bw per day) failed to show any signs of carcinogenicity. There were no research on reproductive toxicity, nor were there any studies on maternal or developmental toxicity, however the study on chronic toxicity found no effects on the reproductive organs. Based on these studies, the EFSA Panel on Food Additives and Flavourings (FAF), established a group ADI of 240 mg/kg bodyweight per day, expressed as tartaric acid, for l(+)-tartaric acid and tartrates.
References:
M. Theron, J. F. Rykers Lues (2011). Organic Acids and Food Preservation. CRC Press.
V. Vaclavik, E. Christian (2014) Essentials of Food Science (4th edition). Springer.
S. Damodaran, K. Parkin (2017). Fennema’s Food Chemistry (5th edition). CRC Press.
J. Provost, K. Colabroy, B. Kelly, M. Wallert (2016). The Science of Cooking: Understanding the Biology and Chemistry Behind Food and Cooking. John Wiley & Sons, Inc.
J. DeMan, J. Finley, W. Jefrrey Hurst, C. Y. Lee (2018). Principles of Food Chemistry (4th edition). Springer


