
If you are used to reading labels off a chewing gum wrapper, you have probably once read the words “mannitol”, “glycerol”, or “sorbitol” in the ingredient list. These ingredients are sugar substitutes in foods that also work as humectants. Chewing gums, candies, and marshmallows are just one of the many food items that contain them. What exactly are humectants? Why are they added?
Humectants have one thing in common—they are hygroscopic. Hygroscopic substances or additives can attract and absorb water. In the food manufacturing industry, they help retain the product’s texture and viscosity, reduce water activity, control crystallization, and more importantly retain moisture.
Table of Contents
HOW DO THEY WORK?
Foods have varying water activity. Water activity is the ratio of the amount of vapor pressure exerted by food to the saturated vapor pressure of pure water at the same temperature. Another meaning of water activity is the amount of free water that is available for various reactions. Microorganisms can use this free water to support their growth. But the lower the water activity, the less moisture is available for microorganisms to grow. This is the reason why foods like dried fish and powders like powdered milk could last for several months to years. Generally, perishable foods have a water activity of higher than 0.9. This is where bacteria thrive. It is why foods such as fresh vegetables, meat, and fish are highly perishable. In order to make a food product more shelf life stable, the water activity must be lowered or controlled.
Further reading: Water Activity (aw) And Food Safety
But not all types of food are meant to be dry, especially intermediate moisture foods (IMF). Think about candies, sodas, diet beverages, chewing gum, and other confections. This is where humectants come in. As hygroscopic substances, they are able to bind the moisture in the food, lowering the water activity. In addition to this, they also absorb moisture in the air.
With the moisture-binding capability of humectant, the food becomes more shelf life stable while keeping them moist. In most applications, humectants function to improve or maintain texture and viscosity, and extends the shelf life. In confectionery, humectants prevent the sugar from crystallizing as a result of moisture loss.
HUMECTANTS IN THE FOOD INDUSTRY
Honey, salt and sugar are one of the oldest humectants food used in food. They are still widely used, especially in home processing (jams and jellies). Traditionally, IMF foods are simply dried until the desired water activity is obtained. But nowadays, processors of IMF foods combine heat treatment such as surface drying and humectants for stronger effect. However, because they may impart flavor and produce solids at certain concentrations, they may negatively alter the product quality. A combination of humectants may be added to prevent these.
In the food industry, food manufacturers use sugar alcohols or polyols as humectants. In chemistry, polyols are compounds that contain multiple hydroxyl groups. Why the name sugar alcohols, though? Well, they are so-called sugar alcohols because their chemical structure resembles the structure of sugar and part is identical to alcohols.

Polyols are a class of sweeteners. They are colorless and odorless. One main difference between sugar and polyols is that polyols are not as readily fermentable as sugar by microbes in the mouth. Hence, they are non-cariogenic—they do not cause tooth decay.
They provide the bulk of sugar, without as many calories as sugar. Manufacturers of fat-free and reduced-fat products use polyols as replacements for the bulk of fat because of their humectant and plasticizing properties.
It is worth noting that not all polyols are good humectants as some are non-hygroscopic. One good example of non-hygroscopic polyol is lactitol. In choosing humectants, it must be:
- Regulatory agency-approved
- Compatible with the nature of the food
- Able to impart no color change in the product
- Effective at reasonable concentrations
- Flavorless at concentrations of use
- Safe
These are four polyols that are used commonly to improve texture and retain moisture in food products.
Sorbitol (E420)
Sorbitol is one of the most commonly used sugar alcohol. And it shares the biggest market among polyols. It appears as a white crystalline powder or a clear, odorless syrupy liquid. Sorbitol is an isomer, along with mannitol, another polyol. It occurs naturally in some fruits such as berries, peaches, plums, apples, and pears.
Commercially, sorbitol is produced by glucose reduction from a starch source such as corn, potato, and wheat. In this method, the aldehyde group of glucose is converted into a hydroxyl group. In the presence of a metal catalyst, the addition of hydrogen converts glucose (C₆H₁₂O₆) into sorbitol C6H14O6).
Sorbitol is around 60% as sweet as sugar, but contains 4 calories per gram. For this reason, many sugar-free and low-calorie foods contain sorbitol. As a humectant, sorbitol is used commonly in baked products like cookies, cakes and breads, and low moisture foods like fruit preserves and peanut butter.
Dental experts support the use of sorbitol as a sugar replacement in food, particularly in chewing gums. Studies have proven that sorbitol is an effective ingredient in preventing caries. This report showed that 14 studies had proven that sorbitol is an effective anti-cariogenic sweetener. Aside from sugar-free and low-sugar foods, oral care products like mouthwash and toothpaste also contain sorbitol.
There have been some reports of individuals who experienced adverse health effects after consuming sorbitol. The common reported discomforts include nausea, cramping, and diarrhea. But these symptoms are very uncommon. And despite these, various regulatory bodies have recognized sorbitol as a safe ingredient in food. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) has not established the acceptable daily intake (ADI) for sorbitol. And the Food and Drug Administration (FDA) has listed sorbitol as generally recognize as safe (GRAS).
Mannitol (E421)
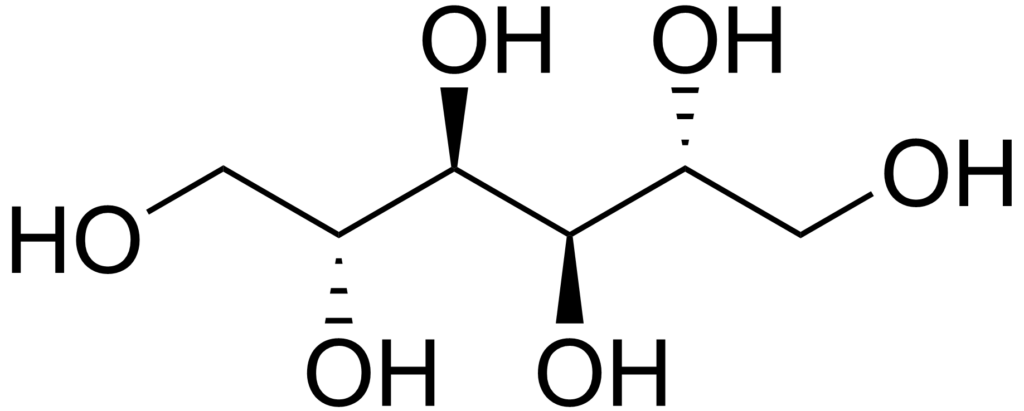
Mannitol is another polyol that is around 60% as sweet as sugar. Each gram of mannitol contains 1.6 calories. It appears as an odorless white crystalline powder or granules.
As already mentioned, mannitol and sorbitol are isomers. The difference between the two is the orientation of the hydroxyl group on carbon 2.
Mannitol can be found in many natural sources such as apples, pears, mushrooms, peaches, cauliflower, and celery. In China, one common source of mannitol is seaweeds. Industrially, there are several ways of producing mannitol. These include hydrogenation, reduction reactions, microbial synthesis, and bioconversion. Owing to its simplicity and robustness, bioconversion is considered the most suitable way of producing mannitol.
Mannitol, as a humectant, is best used as coating for food products such as chewing gums, hard candies, and dried fruits. The reason for this is that mannitol has a very low hygroscopicity. This means mannitol do not readily absorb moisture unless the humidity level in the surrounding reaches at least 98%. Mannitol is also used in reduced or sugar-free confections and energy-reduced products.
Like other polyols, mannitol is resistant to digestion of oral bacteria, preventing the development of dental caries. For this reason, the FDA and the European Commission have allowed products with mannitol to have a health claim on the label that states, “does not promote tooth decay”. However, mannitol-containing products whose consumption may result in a daily ingestion of 20 grams of mannitol shall bear the state, “Excess consumption may have a laxative effect”.
The JECFA has not set the ADI for mannitol.
For the allowable limit of mannitol in certain products such as candies, chewing gums, etc., refer to the FDA.
Glycerol (E422)
Glycerol (glycerine) is a simple sugar alcohol compound. It is a colorless and odorless syrupy liquid. Since glycerol contains three hydroxyl groups, it is miscible and hygroscopic. It contains slightly more calories than sugar (sucrose). Among polyols, glycerol is the most calorie-dense. A teaspoon of glycerols contains about 27 calories, whereas sugar of the same amount contains 20 calories. However, glycerol is around 60% as sweet.
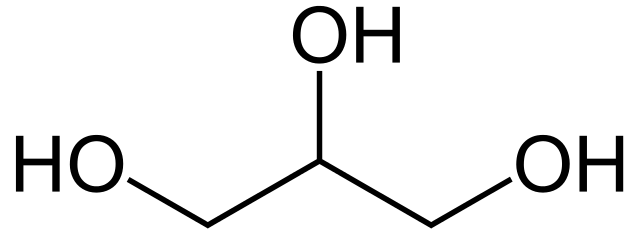
Glycerol occurs naturally by sugar fermentation in beers, wine, and vinegar. However, most of glycerol that we get from food is a product of hydrolysis or breakdown of fats and oils. Because of its sweetness, glycerol also functions as a flavor enhancer. In low fat foods, glycerol works as a filler for foods such as cookies. In baking, adding glycerol prevents icing from setting too hard.
You might also like: 5 Main Functions of Salt (Sodium Chloride) In Food
Studies have shown that consuming glycerol, even in large amounts, does not pose a threat to health. Hence, no acceptable daily intake has been set. Furthermore, the FDA) has declared glycerol as GRAS.
Propylene glycol (E1520)
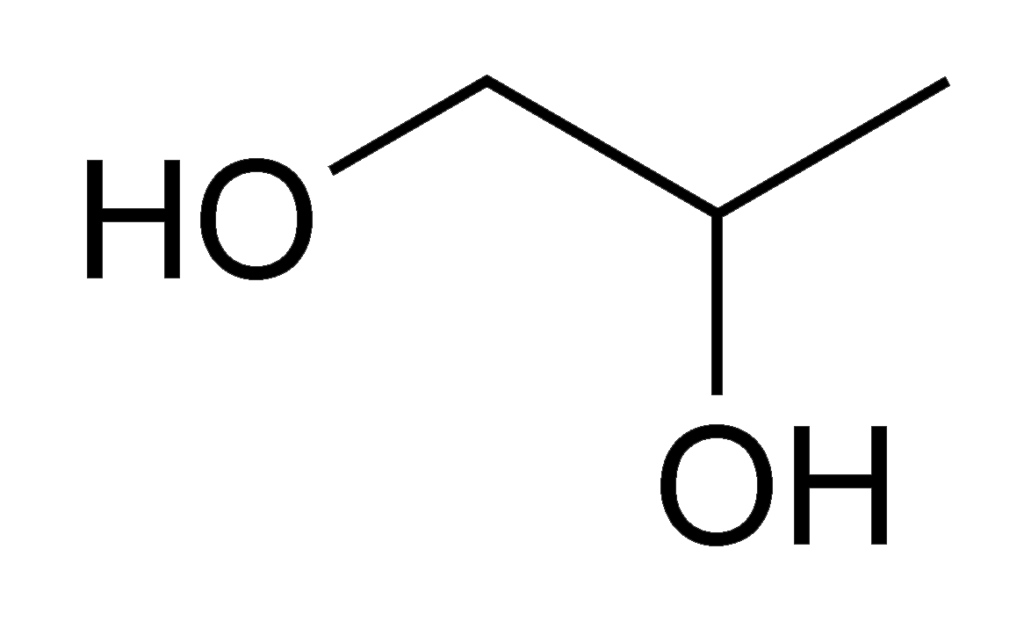
Propylene glycol is a colorless and nearly odorless synthetic syrupy liquid. It contains two alcohol groups—a diol. A gram serving of propylene glycol contains 4 calories.
Propylene glycol may be derived from natural gas, petroleum or vegetable sources. For food production, propylene glycol is usually derived from propylene oxide.
Propylene glycol has multiple uses in the food industry. It keeps certain foods such as marshmallows, coconut, and confections from drying out. Aside from being a humectant, it also acts as a preservative, dough conditioner, emulsifier, and anti-caking agent in foods.
Upon hearing the words “petroleum”, some people may hear it as a bad thing. But ingestion of propylene glycol is not toxic. The reason for this is that our liver can easily metabolize it into normal products. The FDA has deemed propylene glycol as GRAS. However, food manufacturers are only allowed a certain amount of it in their products. Furthermore, JECFA has set the ADI for propylene glycol at 0-25 mg per kilogram of body weight.
Other references:
A. Holban, A Grumezescu (2017). Microbial Production of Food Ingredients and Additives. Academic Press.
M. Shafiur Rahman (2007). Handbook of Food Preservation (2nd edition). CRC Press.
P. Fellows (2000) . Food Processing Technology (2nd edition). Woodhead Publishing Limited.
B. Caballero, L. Trugo, P. Finglas (2003). Encyclopedia of Food Sciences and Nutrition (Second Edition), Academic Press.


