
Have you ever picked up a box of cereal, glanced at the ingredient list, and found a mysterious-sounding compound like ‘trisodium phosphate’ listed there? If you’re like many people, this discovery can leave you with questions, and even a touch of curiosity about what exactly is in your breakfast staple. Rest assured, you’re not alone in your intrigue. Trisodium phosphate, often abbreviated as TSP, is a food additive that occasionally makes its way into breakfast cereals.
In this article, we’ll discuss into the reasons behind the presence of trisodium phosphate in your cereal, as well as its safety. Let’s explore the roles and considerations that come into play when this ingredient finds its way into your daily diet.
Table of Contents
WHAT IS TRISODIUM PHOSPHATE?
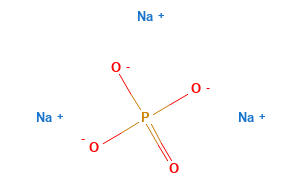
Trisodium phosphate, often abbreviated as TSP, is a chemical compound with the chemical formula Na₃PO₄. Trisodium phosphate consists of three sodium (Na) ions, represented by the Na⁺ cation, and one phosphate (PO₄³⁻) ion, which is a polyatomic anion. The phosphate ion, PO₄³⁻, comprises one phosphorus (P) atom bonded to four oxygen (O) atoms.
In this compound, each sodium ion carries a +1 charge, while the phosphate ion carries a -3 charge, resulting in the need for three sodium ions to balance the charge of one phosphate ion. This is why it’s called “trisodium” phosphate.
TSP is a white, crystalline granule or powder that is highly soluble in water. Its exceptional solubility in water is one of its most notable chemical properties. This solubility is a critical factor in its various applications, as it allows for easy incorporation into liquids. TSP is also a basic or alkaline compound. This means it has a high pH level or alkalinity (between 11.5 and 12.5) when dissolved in water.
Its remarkable solubility in water and basic properties make it valuable in a range of applications, from food processing to cleaning and industrial processes.
HOW IS IT MADE?
Trisodium phosphate (TSP) is typically synthesized through a chemical reaction involving sodium hydroxide (NaOH) and phosphoric acid (H₃PO₄). In this manufacturing process, sodium hydroxide, commonly known as caustic soda, and phosphoric acid serve as the fundamental raw materials.
The chemical equation representing this reaction is 3 NaOH + H₃PO₄ → Na₃PO₄ + 3 H₂O. This reaction yields trisodium phosphate (Na₃PO₄) and water (H₂O).
Following the chemical reaction, the resulting trisodium phosphate product may exist in a solution form. To obtain solid trisodium phosphate, a crystallization step is typically employed. The solution is allowed to cool and evaporate slowly, which induces the precipitation of trisodium phosphate crystals. Subsequently, the trisodium phosphate crystals are separated from the residual liquid, dried, and prepared for packaging, facilitating distribution and utilization.
The precise manufacturing process may vary depending on the specific grade and intended application of trisodium phosphate. Additionally, trisodium phosphate can assume various hydrate forms, impacting the water content in the final product.
TRISODIUM PHOSPHATE FUNCTIONS IN CEREALS
Trisodium phosphate is a common breakfast cereals addition that serves many purposes as an acidity regulator, color stabilizer, and texture enhancer.
TSP, as an alkaline chemical, effectively modulates the acidity of cereals. A food product’s acidity or alkalinity has a significant impact on its flavor profile. Cereals with high acidity can develop an unpleasant tartness, especially if they incorporate items that naturally add to acidity, such as fruits or yogurt. However, in cereals, such acidity might have a negative impact on flavor.

To address this issue, manufacturers incorporate TSP into cereal production. TSP is an alkaline compound, and when added to acidic cereals, it effectively neutralizes the excess acidity. By doing so, it balances the pH and ensures that the cereal maintains a more harmonious and pleasing flavor profile. This adjustment in acidity contributes to a cereal that is not only more palatable, but also suitable for a broader range of taste preferences.
Acidity also has an impact on the color of cereals. Cereals frequently include various ingredients, such as fruits, nuts, or grains, each with its distinct color. Over time, the colors of these ingredients can fade or leach into the cereal due to acidity and chemical changes. Additionally, during processing and storage, cereals are exposed to various conditions that can further contribute to color degradation, making them appear dull or unappealing. However, regulated acidity due to the presence of TSP prevents this from occurring.
As cereals consist of ingredients with varying compositions, there is a potential for these ingredients to separate, resulting in uneven distribution within the cereal and inconsistent textures. With the addition of TSP, the ingredients are bound together. Consequently, this ensures even distribution of ingredients, guaranteeing a consistent taste and texture with every bite.
SAFETY AND MISCONCEPTIONS
Concerns and fears about trisodium phosphate (TSP) in cereals, like with many food additives, often stem from a combination of factors, including misconceptions, misinformation, and a general desire for natural and minimally processed foods.
TSP is not a household ingredient, and its name might sound unfamiliar and artificial to some consumers. Some people even persuaded consumers on various social media platforms to not consume cereals because it is a harmful chemical that is used in cleaning agents and paint thinners.
Well, TSP is allowed, and commonly added to cereals and other food products for a reason. In the United States, the Food and Drug Administration (FDA) has classified it as Generally Recognized as Safe (GRAS). This indicates that TSP is considered safe for consumption when used within established limits. It’s essential to understand that many substances can be harmful if consumed in excessive amounts, and TSP is no exception. The safety of TSP in food is not solely based on its inherent properties, but also on the dosage or quantity used.
In the European Union, trisodium phosphate (TSP) is known as food additive E399. The European Food Safety Authority (EFSA) has approved TSP as a safe food additive. After reviewing the evidence, EFSA concluded that phosphates have low acute oral toxicity and are not genotoxic or carcinogenic. EFSA has set an acceptable daily intake (ADI) for phosphates of 40 milligrams per kilogram of body weight per day. The ADI is the maximum amount of a substance that a person can safely consume daily over a lifetime without experiencing adverse health effects.


