
Many food preparations use egg whites to create foam. Foams are those substances that form by dispersing or trapping gas (bubbles) in a solid or liquid. Foams generally give food volume and distinctive mouthfeel. The liquid phase may be a simple dispersion, like in egg white, which is a dilute protein dispersion, or it may be complex, such as those that contain emulsified fat droplets or ice crystals.
As one might expect, foaming agents possess similar characteristics as an emulsifier. They are able to decrease interfacial tension, adsorb at the interface, and create a stable interfacial film that prevents rupture. Many proteins are good at producing foams. However, proteins from egg whites are superior foaming agents. Foods such as angel food cake, meringue, soufflés, and other baked goods rely on egg white foam to become light and airy.
Have you ever wondered how foam formation occurs in egg whites? To better answer this, let’s see what egg whites are made up of.
EGG WHITES AND PROTEINS
Egg white is about 90% water and 10% protein. Proteins consist of long chains of amino acids that fold and curl into spherical tangles. Over half of the protein in egg whites are ovalbumin (54%). Other proteins include ovotransferrin (12%), ovomucoid (11%), ovomucin (3.5%), and lysozyme (3.5%). These have been identified as functionally important proteins. Like for example, lysozyme protein can be used as a preservative because of its antimicrobial properties. Ovotransferrin functions as a metal transporter, preservative, or anti-cancer agent. The separation methods for these proteins have already been established. However, the preparation methods for commercial use are still under development.
You might also like: Why You Should Not Use Pasteurized Egg (Whites)
Without the need for separation from the egg white, these proteins have one important function in cooking and food preparation—foam formation.
But there should be water, which is 90% in egg whites. With just water alone, whipping egg white would just produce bubbles that quickly pop. There is just too much attraction between the water molecules to create a bubble film. There is a need to reduce the attraction between these molecules. This is where the egg white proteins come in.
By whipping the egg whites, there is physical stress that leads to denaturation and coagulation. Gas bubbles (air) also get trapped in the liquid, together with the unrivaled proteins, producing foam. Protein denaturation is discussed in more detail here.
WHAT EXACTLY HAPPENS DURING FOAM FORMATION
To produce foam, an equipment, mainly beater or whisk, is necessary to induce physical stress. As the whites are whipped or beaten, air is incorporated into it. This produces tiny air pockets that get trapped by proteins, increasing volume.
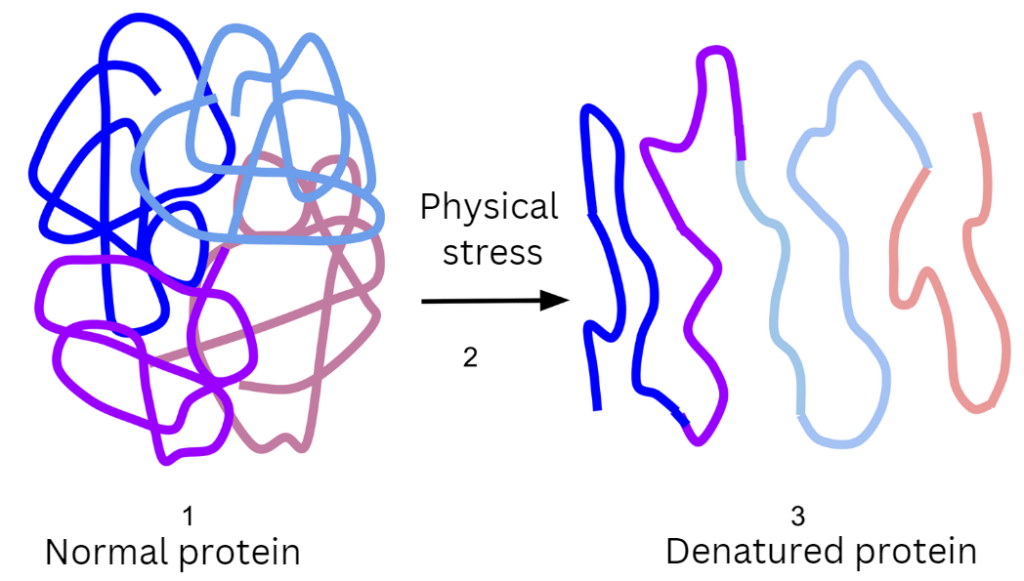
The whipping also leads to denaturation of the proteins. Protein denaturation is the physical changes of protein molecules from their natural state as a result of exposure to abnormal conditions (such as increased temperature, pH, or physical stress). In the case of beating egg whites, the surface changes unravel and stretch the proteins out. Egg whites easily create physical bonds with each other. And egg white proteins consist of hydrophilic (water-loving) and hydrophobic (water-repelling) ends. Because of this, the proteins line up to form a continuous solid matrix of proteins that holds water and air in place, producing a stable, viscoelastic interfacial film.
You might also like: Is It Safe To Eat Eggs With Blood Spots?
EGG WHITE FOAM STABILITY
Some proteins in egg whites are glycoproteins, which consists of protein and carbohydrate chains. When they adsorb at the interface, the carbohydrate chains orient toward the aqueous phase. A hydrophilic, they bind water and improve the viscosity of the liquid. This helps to minimize drainage, thereby contributing to foam stability.
Foam stability is crucial when manufacturing foam-containing food products. The key to producing highly stable foam is increasing the bonds or connection between proteins. These proteins are predominantly water-loving or hydrophilic. So introduction of any fat will definitely affect adversely the ability of egg whites to produce foam. In most cases, this happens when yolk, which contains all the fat within an egg, has been added by accident. So it is very important to carefully separate the yolk from the white. Another reason is the use of greasy utensil or equipment during food preparation. To prevent this, utensils, especially bowls for whipping, should be made from metal or glass. The reason for this is that fat readily sticks to utensils made of plastic.
You might also like: 5 Truths You Need To Know About Eggs
Although whipping turns egg whites into foam, overwhipping them affects foam stability. Overwhipped proteins encourage uncurling excessively, leading to too many bonds that may form. This draws the proteins closer to one another, squeezing and pushing the bubble-trapped air out instead of containing it. The result is grainy and dull egg whites that may eventually collapse. One way to prevent this is to add a small amount of acid (lemon juice, cream of tartar, or vinegar). The acid should aid in stabilizing the foam by allowing the proteins to unravel a bit and tangle along with other lightly unraveled proteins.
To whip egg whites without difficulty, whip them at room temperature. The decreased surface tension makes whipping easier since the proteins expand readily.
References
J. Provost, K. Colabroy, B. Kelly, M. Wallert (2016). The Science of Cooking: Understanding the Biology and Chemistry Behind Food and Cooking. John Wiley & Sons, Inc.
V. Vaclavik, E. Christian (2014). Essentials of Food Science (4th edition). Springer.
M. Gibson (2018). Food Science and the Culinary Arts. Academic Press.
N.A. Michael Eskin, F. Shahidi (2013). Biochemistry of Foods (3rd edition). Academic Press.
.


Pingback:The Ratio To Remember When Substituting Aquafaba For Eggs – Plant Based Weight