
In 1912, French scientist Louis Camille Maillard was looking for ways to synthesize proteins in vitro. During his work, the color changes and the odors produced led him to describe the reactions that occur between reducing sugars and amino acids, first with glycine and glucose. Then Maillard described the formation of dark-colored compounds, which he named melanoidins. Later on, he established the order of reactivity for the types of sugars that react with different amino acids. He eventually wrote and published 14 articles on reactions between sugars and amino acids. However, the mechanisms or the specifics on how each reaction takes place remained a mystery until the 1950s. It was in 1953 when American chemist John E. Hodge published a paper describing a mechanism for the reaction. Literature started referring to this complex set of reactions as Maillard reaction by the 1950s.
Let’s further discuss.
Table of Contents
WHAT IS THE MAILLARD REACTION?
The Maillard reaction is a complex set of reactions between sugars and amino acids at elevated temperatures. Maillard reaction, along with caramelization, is a type of non-enzymatic browning; they produce brown pigments in food without the presence of enzymes. Since the Maillard reaction requires amino acids, it takes place in most protein-containing foods. It is responsible for the flavors and enticing browning in breads, steaks, toasted nuts, chocolate, coffee, and French fries. The Maillard reaction can form several hundred of flavor compounds. This depends on many variables such as the cooking temperature, cooking time, and the components in the food.
You might also like: The Science Behind Caramelization
Through Maillard reaction is one way of producing artificial flavors. For this reason, the Maillard reaction is of great importance not only in cooking, but in food manufacturing as well.
WHAT HAPPENS DURING THE MAILLARD REACTION?
The Maillard reaction is all about heat, sugars, and proteins. In order for the reaction to place, heat is required for amino acids and the sugars to react together. This typically occurs at 284ºF (140ºC). At this temperature, protein-rich foods turn brown. We can observe them in the crust of roast pork meat, French fries, and seared steak. When coffee is roasted, melanoidins account for up to 25% of roasted coffee beans. But interestingly, the browning in foods is just a part of the Maillard reaction. While the food cooks, the amino acids and sugars react and combine, forming new flavor compounds.
The fused molecules then collide into others in order to combine, separate and reform. This way, more complex flavor molecules are able to form. Their movement is faster as the temperature rises to around 302ºF (150ºC), forming more compounds twice as fast. The flavor enhancements peak at around 320°F (160°C). At this point, flavor profiles such nutty, meaty, caramel-like, and malty could be perceived.
Different flavor compounds can be created during the Maillard reaction. This depends on the amino acid or sugars present. For example, the sulfur-containing amino acid cysteine reacts with sugars to form thiazoles and thiophenes, the compounds responsible for the roasted meat flavor in red meat.
Cooking food at around 356°F (180°C) or higher should be avoided though. If food reaches this temperature, pyrolysis reaction or decomposition will start. This will make the food to turn black, aromas get destroyed, leaving only acrid and bitter flavors. Furthermore, the macronutrients carbohydrate, protein, and fat, will start to break down, producing harmful substances. And some of them are possibly carcinogenic.
A closer look at the chemistry behind the Maillard reaction
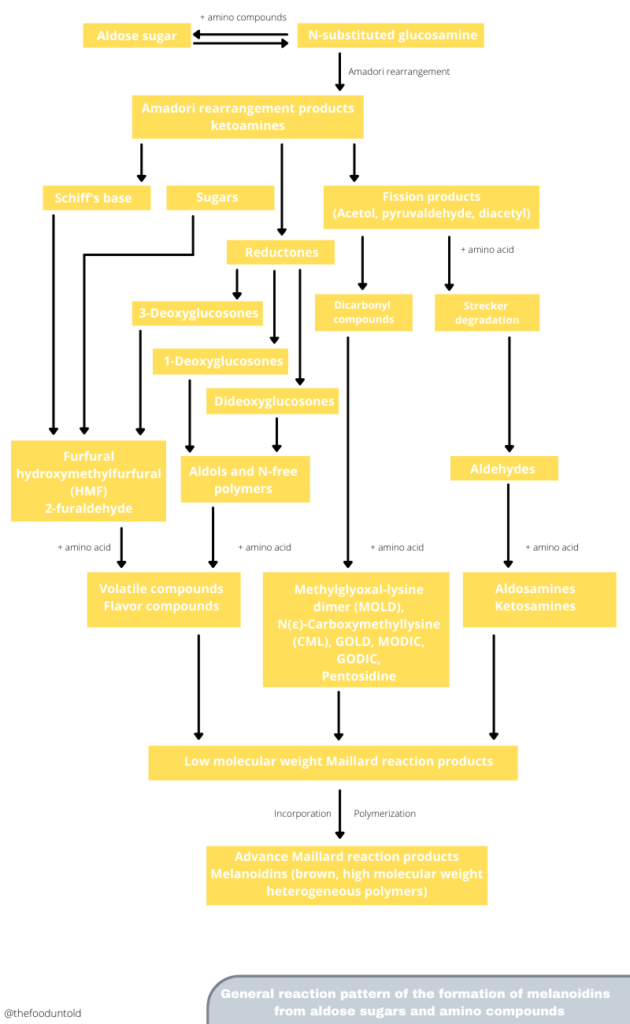
Let’s take a closer look at what happens during the Maillard reaction this time.
It all starts with the carbonyl group (C═O bond) of the sugar reacting with nitrogen of an amino group of amino acids and proteins. An amino group is composed of one nitrogen atom and two hydrogen atoms (NH2). Now, this is the first stage of the chain of reactions, as identified by Hodge. The reaction between the two produces an unstable glycosylamine compound.
In the second step, the glycosamine compound undergoes isomerization or Amadori rearrangement, forming ketosamines (and Heyns arrangement for ketoses). Then, a series of reactions which lead to the formation of the Amadori product follows.
Next, the rearrangement, dehydration, decomposition, and/or reaction of Amadori intermediates occur. These reactions produce various compounds, which include furfural compounds, reductones, and dehydro reductones. These also include short chain hydrolytic fission products such as acetol, diacetyl, and pyruvaldehyde, which undergo Strecker degradation. Strecker degradation converts an α-amino acid by oxidation to the corresponding aldehyde containing the side chain. Aldehydes contribute to the aroma of cocoa, peanuts, bread and other roasted foods. The dicarbonyl compounds also react with more amino acids to form more Maillard reaction products.
In the third and final step, The Maillard intermediary products react to form heterocylic flavor compounds. The reactive carbonyls (HMF, furfurals and other compounds) amino group-containing compounds polymerize. This results in the formation of brown, low molecular weight pigments called melanoidins. Melanoidins are responsible for the dark colors of beer, roasted coffee, browned meat, and bread.
Reducing sugars
While it is true that the Maillard reaction requires sugars in order to take place, not all sugars work well in the reaction. When talking about the Maillard reaction, a particular type of sugars called “reducing sugars” are necessary. Reducing sugars are sugars that contain either a free ketone (–CO–) or aldehyde (–CHO) group. They can act as a reducing agent in chemical reactions, which, of course, include the Maillard reaction. Why the name reducing sugar is because of the reaction that takes place between the C═O and another compound. If the conditions favor, the C═O will react and make the other compound be reduced in its electron state. To simply put, they are sugars that donate electrons to another molecule.
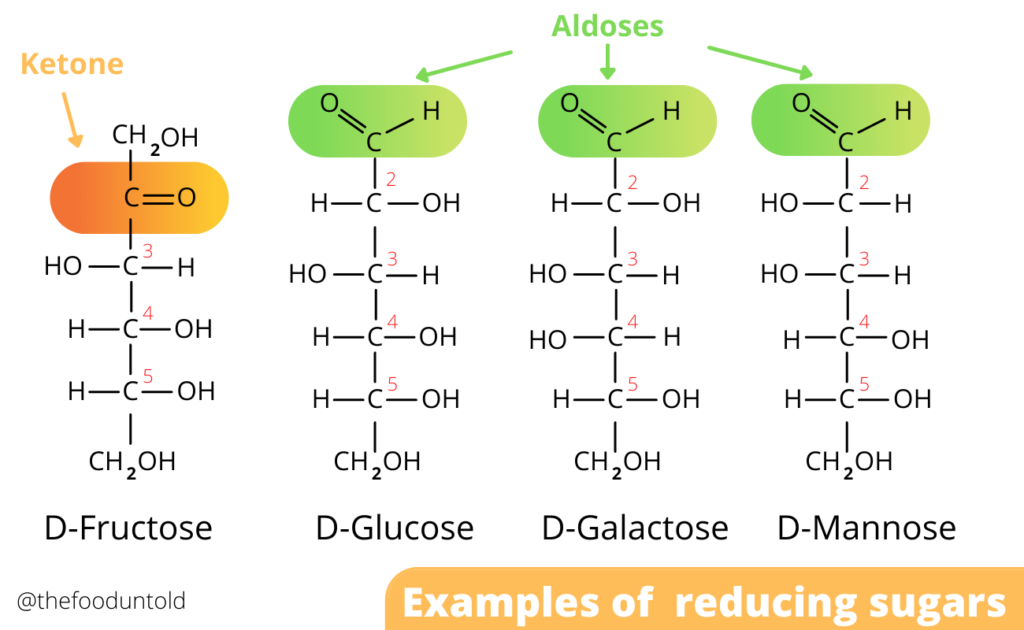
All monosaccharides, which include glucose, galactose, and fructose, are reducing sugars. While many disaccharides, which include maltose and lactose, also have a reducing form. This happens only when one of the anomeric carbons convert into the open chain format. Sugar molecules can have two different structures: an open chain and ring or closed form.
You might also like: Caramel Color (E150): What Is It As A Food Additive?
The ring opening process is what produces a C═O at the anomeric carbon. This carbonyl group that formed upon ring opening can participate in the Maillard reaction. In some sugars, ring opening does not occur. In the case of sucrose, the anomeric carbon is not free because it is utilized to hold the simple sugars glucose and fructose together. Hence, the anomeric carbon does not ring open the structure for sucrose to react in the Maillard reaction.
Sugar alcohols or polyols are sugar substitutes in low-calorie foods. They also do not participate in the Maillard reaction. This is why sorbitol does not produce browning when used in baking or cooking.
Amino acids and proteins
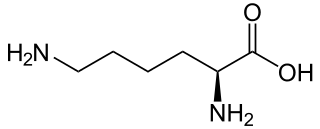
Amino acids are the building blocks of proteins. These are the second reactants involved in the Maillard reaction. At the start of the reaction, the amino nitrogen bonds to anomeric carbon of the reducing sugar. The combination of the nitrogen of amino groups and the reducing sugar is referred to as a dehydration reaction. It is characterized with a loss of a water molecule. The product of dehydration rearranges, forming an Amadori compound
There are 20 amino acids. Each of these has an amino group. The amino acid lysine has a side chain, which contains an additional amino group. Lysine is also the most reactive amino acid. It browns superbly in foods rich in ribose. Lysine-containing proteins in baked foods produce rich flavor and dark color when toasted. The amino acid cysteine also works well with ribose. The reaction between these two produces over 200 different volatile compounds (strong meaty flavor and aroma). Aside from lysine, glycine, tryptophan and tyrosine are also high-browning amino acids.
PRODUCTS OF THE MAILLARD REACTION
The Maillard reaction can produce hundreds of flavor and aroma compounds, which can then move in countless ways to form more compounds. Here are some.
| COMPOUND CLASS | FLAVOR/AROMA | FOOD |
| Acylpyridines | Cracker-like | Cereals |
| Alkylpyrazines | Roasted, nutty | Coffee |
| Alkylpyridines | Astringent, bitter, burnt, green | Malt, coffee, barley |
| Furans, furanones, and pyranones | Caramel-like, | Heated foods |
| Pyrazines | Roasted, toasted, cooked, baked cereals | Heated foods |
| Pyrroles | Cereal-like | Coffee, cereals |
| Oxazoles | Nutty, sweet, green | Coffee, cocoa, meat |
| Thiophenes | Meaty, roasted | Cooked meat |
FACTORS THAT AFFECT THE MAILLARD REACTION
There are several factors that affect the rate of the Maillard reaction. They also dictate the aroma or flavor compounds that form.
The reacting sugars and amino acid
The nature of the reacting sugars and amino heavily influence the reaction velocity as well as the pattern in the Maillard reaction. This is why each food may develop a different browning pattern.
Among the 20 amino acids, lysine is the most reactive because of its free ε-amino group. However, in most food proteins, since it is the limiting essential amino acid, its destruction may reduce the nutritional value of the protein significantly. Ammonium ions also react more easily with reducing sugars than amines. And while amino acids, peptides, and proteins may undergo the Maillard reaction, the reactivity of the proteins is largely due to the presence of the free ε-amino group of lysine. And the thiol group of cysteine and guanidyl group of arginine may react as well.
Foods that are rich in reducing sugars are also very reactive. This explains why lysine found in milk gets destroyed more readily that that in other foods. In terms of functional group classification, aldoses (with an aldehyde group) are more reactive with amino acid than ketoses (with a ketone group). Some studies have revealed that D-fructose (a ketose) is more reactive in the Maillard reaction than D-glucose (an aldose). While a non-reducing sugar, sucrose may be broken down into fructose and glucose at elevated temperature and still contribute to Maillard reactions.
One quick way to increase the rate of the Maillard reaction is adding a reducing sugar (such as fructose and glucose) or protein (milk, egg).
Moisture
Water slows down the rate of the Maillard reaction. This is partly because water absorbs water during heating. With a significant amount of water in food, the temperature of the food will stay at water’s boiling point of 212 °F (100 °C).
With heating, water content at the surface of the food decreases, drying the surface out, and increasing the temperature necessary for the Maillard reaction to occur. Then moisture is drawn out from within, refueling the surface with Maillard reaction precursors monosaccharides and amino acids. This is why the Maillard reaction is at the strongest on the surface, like of a joint of beef. In baked products, the surface is brown and dry, but the middle is moist and unreacted.
Cooking methods such as frying, baking, and grilling produce foods with a brown surface. Moist cooking methods such as poaching, boiling, and steaming, on the other hand, only produce cooked and moist foods. These foods will not go brown nor will it have the flavors of the Maillard reaction.
pH level

The pH level (acidity or basicity) strongly influences the rate of Maillard browning in food. The more basic or alkaline the food is, the faster the rate of browning. Generally, the rate of browning can increase several folds when the pH is above 7 (neutral pH). The speed of browning can be easily observed when pretzels are dipped in lye and then cooked. Lye is basically a solution of potassium or sodium hydroxide—a strong basic or alkaline.
Dipping in lye creates an alkaline pH on the surface of the pretzel. This allows the Maillard reaction to occur even at lower temperatures. Plus, the baking time is reduced without sacrificing the development of flavor and browning.In some recipes, baking soda or sodium bicarbonate, is not a required ingredient, but is often added during cooking to help kickstart the Maillard reaction.
But how does exactly pH affect the rate of the Maillard reaction?
In alkaline conditions, the sugar molecules are in the ring-opened form. Therefore, the anomeric carbon is able to react with the amino group. In acidic conditions, on the other hand, the reducing sugars are in the ring conformation. Hence, they are unable to react. Furthermore, the amino group becomes a protonated ammonium ion, which is unreactive. Raising the pH level removes the proton from the amino group, making the reaction to occur.
Temperature
The Maillard reaction occurs generally when food is heated. But this does not mean sugars and amino acids do not react at lower temperatures because they do. But not at an appreciable rate. An aged champagne would still develop a yellow color because of the proteins and sugars in the grapes undergoing the Maillard reaction. But since the temperature is cool, the reaction responsible for the formation of the yellow Maillard compounds would be very slow.
In most foods, especially protein-rich ones, the Maillard reaction starts producing aroma compounds and melanoidins at 284ºF (140ºC). The temperature is necessary to increase the number of collisions and the number of molecules with sufficient energy to react together. As the temperature rises, molecular changes continue, creating more flavor and aroma compounds.
THE POSSIBLY CARCINOGENIC ACRYLAMIDE
Maillard reaction is a very important process in foods. It gives them better taste, flavor, and color.
However, like we have already mentioned earlier, cooking food at high temperatures triggers the formation of harmful substances. Perhaps, the most feared toxic by-product of Maillard reaction is acrylamide. When polymerized, acrylamide is used by scientists to analyze protein size and also for producing plastic. Acrylamide is also present in tobacco smoke.
But while studying the Maillard reaction in 2002, scientists found small amounts of acrylamide in foods. Levels of acrylamide were higher heavily processed food, including potato chips and French fries. Further studies have also revealed that acrylamide is also present in biscuit, cereals, crackers, and bread. This compound forms with heating of food at above 284 °F (120 °C) by frying, baking, and broiling.
You might also like: Korean Study: Overcooking With Air Fryers Creates Toxic
Acrylamide is not a natural compound. But a product of the Maillard reaction between the amino acid, mostly asparagine, and some reducing sugars. Since its discovery in foods in 2002, numerous studies have been conducted to determine its potential as a human carcinogen. In rodent studies, the results revealed an increase in the likelihood of developing tumors and gene mutation. The experts of European Food Safety Authority (EFSA) agree that acrylamide in food increases the risk of developing cancer. And in 2010, the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA) concluded acrylamide to be a human health concern. However, regulatory agencies have recommended further studies to fully determine the full impact on the human diet.
Other references:
deMan, J. Finley, W. Jeffrey Hurst, Chang Yong Lee (2018). Principles of Food Chemistry (4th edition). Springer.
V. Vaclavik and E. Christian (2014). Essentials of Food Science (4th edition). Springer.
M. Wallert, K. Colabroy, B. Kelly, J. Provost (2016). The Science of Cooking: Understanding the Biology and Chemistry Behind Food and Cooking. John Wiley & Sons, Inc
G. Mark (2018). Food Science and the Culinary Arts. Academic Press.
S. Damodaran, K. Parkin (2017). Fennema’s Food Chemistry (5th edition). CRC Press.
S. Farrimond (2017). The Science of Cooking: Every Question Answered to Perfect Your Cooking. DK Publlishing.



Pingback:Korean Study: Overcooking With Air Fryers Creates Toxic Substance - Alternative Health Specialists
Pingback:Is Your “Healthy” Air Fryer Emitting Cancerous Chemicals? Here Are The Safest Options And Those To Avoid At All Costs – The Wellness Thesis