Emulsifiers are one of many additives that are used extensively in the food manufacturing industry. But what exactly are emulsifiers, really? Would food product that usually contain them be exactly the same without them? Well, basically, emulsifiers are food additives that produce and maintain an emulsion. And an emulsion is a mixture or two or more liquids that normally do not mix together.
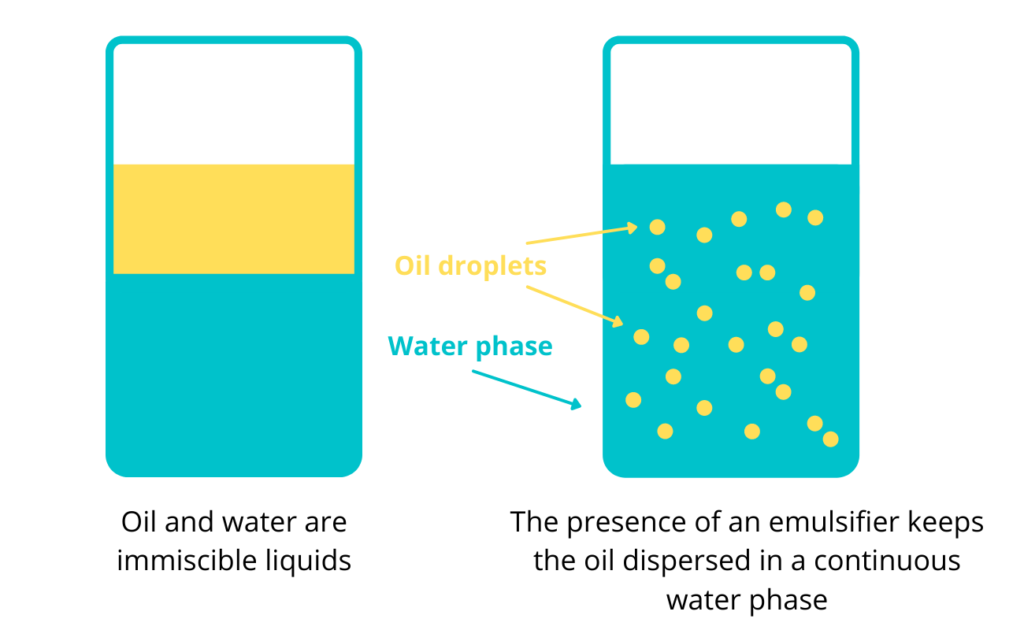
To better explain what exactly an emulsion is, let’s have oil and water as an example. These two liquids are not miscible—they do not form a homogeneous mixture. Even if we try to mix them, the water molecules will only attract with each other and the oil molecules will stick together. And since water molecules pack closer together, the oil will only find itself sitting on top of the water. This is where emulsifiers come in. They work to stabilize emulsions to keep the liquids from separating. They do this by breaking up either the water or oil phase into small droplets that remain dispersed throughout the other liquid.
Common emulsion is oil and water in salad dressings, shortenings, margarine, and ice cream. How do emulsifiers exactly work?
Let’s discuss further.
HOW EMULSIFIERS WORK
Emulsifiers are a part of a group called surfactants or surface active agents, which also include foaming agents, detergents, and dispersants. They mainly help lower the tension between two phases of matter to achieve a certain goal. Detergents, for example, enable water molecules to spread more easily, allowing it to clean dirt from the surface. In the case of emulsifiers, they decrease the tension between two liquids.
While oil and water are immiscible, emulsifiers keep them from separating. They are able to achieve this because they contain both a hydrophobic molecular end and a hydrophilic end. A hydrophobic molecule repels water, but are attracted to oil (“oil loving”). Whereas a a hydrophilic molecule has a tendency to dissolve in and mix with water (“water-loving”). The presence of both types of molecules allows an emulsifier to act as a binding agent between oil and water. This, in return, produces a homogeneous emulsion. Generally, emulsions have minimal stability. However, surface active agents and other similar substances can help enhance their stability.
Liquids or emulsions in food can be oil-in-water (mayonnaise) or water-in-oil. An example of a water-in-oil emulsion is margarine. The continuous phase or the surrounding liquid here is oil, while the dispersed phase is water, which is in the form of small droplets. Without an emulsifier, instead of water dispersing in oil, it will separate to form layers of oil and water. And that is not what a margarine should look like, right?
Emulsifiers also have other functions in food. They are able to form complexes with other food components. One good example of this is the amylose-complexing effect of emulsifiers. This effect is beneficial for improving the shelf life of baked products (anti-firming effect) and modifying the physical properties of starchy foods such as potatoes and pasta.
FACTORS THAT AFFECT EMULSION STABILITY
One of the many challenges that the research and development of the food industry face is how the ingredients would interact with each other. This is especially true once they undergo processing or storing. Here are the factors that affect emulsion stability.
Emulsifier
There is no wonder why the type of emulsifier itself is the main factor that affects emulsion stability. Basically, emulsifiers that produce stable interfacial films also produce stable emulsions. There must be also an adequate concentration of emulsifier in order to coat the surface of all the droplets.
Droplet size
Speaking of droplets, their size also affects emulsion stability. Large droplets tend to coalesce, where two or more droplets merge during contact. Moreover, oil and water have a significant density difference—water is more dense (heavier) than oil. So oil floats above the water. But large oil droplets rise through the emulsion more easily, creating a stronger emulsion closer to the surface. This will eventually cause the emulsion to break.
Ph and ionic strength
pH or acidity also affects emulsion stability. By changing the pH by adding acid, it may reduce the stability of the interfacial fil,. Adding salts changes the ionic strength (concentration of ions), which may also reduce the stability of the interfacial film.
Viscosity
The viscosity of the emulsion also affects stability. The thicker the emulsion, the slower the molecules move within the system. Hence, the longer it will take for the two phases to separate. One common way to modify the viscosity is the addition of a thickener such as pectin, gums, or gelatin. The addition of gums in salad dressings may already form a permanent emulsion without the need for an emulsifier such as egg yolk.
It is worth noting that gums are added as stabilizing agents, not as emulsifiers because they are hydrophilic. But their ability to increase the viscosity of the system helps reduce the number of collisions between droplets. This slows down or prevents separation of emulsions.
Storage and handling
During storage or handling, some emulsions can also be affected negatively. Emulsions are delicate systems since they contain immiscible liquids. Hence, improper handling can cause breakage of the emulsion.
Temperature
Another factor that affects emulsion stability is temperature. Generally, temperature affects the properties of water, oil, as well as interfacial films, and surfactant solubilities. In a warm temperature, oil droplets become more fluid. And coalescence is more likely to occur. These reduces the stability of emulsions.
Refrigeration temperatures, on the other hand, solidify oil droplets, enhancing emulsion stability. However, freezing temperatures are not ideal for most emulsions. The reason for this is that emulsions do not survive freezing temperatures. Proteins at the interface become denatured at freezing. Furthermore, ice crystals that form tend to rupture the interfacial film. Before being subjected to freezing, emulsions are often added with gums to enhance their stability.
A FEW COMMON APPLICATIONS OF EMULSIFIERS IN FOOD
The oldest emulsifier known to man is beeswax. It was when the Greeks first used it as an emulsifier in cosmetic products. Then in the early 19th century, food manufacturers started using egg yolk, the first emulsifier to be used in food. Egg yolk owes most of its emulsifying capability to its phospholipid lecithin content. Lecithin is an amphiphilic fatty substance—able to attract both water and fat. While an effective emulsifier, egg yolk has a rather short-term stability.

For this reason, food manufacturers had to find a better alternative to egg yolk. They found it in the form of lecithin derived from soybeans.
However, the most significant development came in the 1930s with the introduction of mono- and di-glycerides of fatty acids. Initially used in margarine, these synthetic emulsifiers later extended its uses, which include ice cream and baked products such as breads and cakes. Other synthetic emulsifiers were later introduced for use commercially in the late 20th century. Today, common emulsifiers include mono- and diglycerides of fatty acid, guar gum, carageenan, and polysorbates. Here are some of their common food applications.
Ice cream
Ice cream is a mixture of solids, partially frozen milk fat and ice; liquid, unfrozen cream and water; and gas, pockets of air trapped in freezing mixture. All these three phases form a colloid. But ice cream is not only an emulsion, but a foam as well. And it contains ice crystals and unfrozen aqueous phase, whose freezing point is depressed by freeze concentration sugars, salts, and polysaccharide stabilizers.
| INGREDIENT | WEIGHT PERCENT |
| Fat | 10.0 |
| Milk solids, non fat | 11.0 |
| Sugar | 13.0 |
| Stabilizer | 0.2 |
| Emulsifier | 0.5 |
| Water | 65.3 |
In ice cream manufacturing, the process is relatively simple. The ingredients such as milk, sugars, and milk solids are mixed. The table on the left shows the representative composition of ice cream. After mixing, it is then treated with heat to destroy the pathogens, and then homogenized. Homogenization helps reduce the fat droplets, which prevents churning of the fat upon whipping.
The incorporation of emulsifiers in ice cream produces a product that whips more easily, has a smoother body and texture, is drier, has better meltdown resistance during consumption, and with minimized shrinkage during storage.
You might also like: Emulsifiers Used In Ice Cream
Common emulsifiers in ice cream production are polysorbates (E432, E436), and mono and diglycerides (E471). The reason for this is their relative abilities as emulsion destabilizers, or as foam-forming agents. Polysorbates are better at displacing protein from the oil/water interface than mono and diglycerides. And therefore are better emulsion destabilizer. Mono and diglycerides, on the other hand, are better foaming agents, hence aid in the formation of the initial foam prior to fat-droplet agglomeration at the air/water interface. Other emulsifiers used in ice cream include lactic acid esters (E472b), lecithin (E322) and propylene glycol esters (E477).
Baked products
According to a 1996 study, bakery products are the largest users of emulsifier. The bakery industry accounts for 50% of the food emulsifiers market. Emulsifiers have multiple functions in baked products. But unlike in other food products, emulsification of oil in water is of secondary importance. Perhaps, emulsifiers hold more value in protein strengthening, starch complexing, and aeration.
You might also like: What Is Potassium Bromate (E924) And Why Many Countries Have Banned It In Baked Products?
Shortening is one of the key ingredients in baking. When added and mixed into a hydrated dough, it interrupts in the development of the gluten network. Or the structure is “shortened”, to simply put. This produces a product that is tender, has rich flavor and uniform aeration. Common shortenings include margarine, butter, margarine, and lard. Adding an emulsifier to the shortening promotes the emulsification of the shortening in the batter or dough.
Emulsifiers used in breads and buns include lecithin, diacetyl tartaric acid esters of monoglycerides (DATEM), polysorbate 60, calcium stearoyl lactylate (CSL), sodium stearoyl lactylate (SSL), and mono and di-glycerides. Among these, monoglycerides in their many forms are the most used emulsifiers in baked products, especially yeast-baked products, as well as icings, cakes, and other applications. Cakes with monoglyceride-containing shortening have better aeration and sugar holding capacity. Monoglycerides also retard staling rate, improving the shelf life of breads.
Infant nutritional products
These types of products are specially formulated for infants as well as young children. They come several forms: concentrated liquid, ready-to-feed liquid, and powder reconstituted for consumption. A stable oil-in-water emulsion is an important step in the manufacture of these products. Normally, this is achieved by thorough homogenizing the oil phase in an aqueous phase, which consists mainly of carbohydrates, proteins, vitamins, and minerals. The oil phase usually consists of a blend of vegetable oils that include coconut, palm, sunflower, and soybean oil. The formation of a stable oil-in-water emulsion is a common prerequisite of both liquid and powder processes.
You might also like: Fortified Vs Enriched: What’s The Difference?
In liquid products and products produced by the wet-mixing/spray drying system, a fluid fat blend is dispersed and emulsified in an aqueous system, which contains carbohydrates, proteins, minerals, and emulsifiers. In the case of dry blended formula, the fat has already been emulsified within a system, usually one more more protein sources.
The emulsifier ingredients in infant nutritional products can be classified into two: the protein and non-protein emulsifiers. Bovine milk proteins are very common in infant nutritional products. Other protein emulsifiers include demineralized whey, milk protein isolate, soy protein isolate, and calcium caseinate. The non-protein emulsifiers are low molecular weight surfactants. Common protein emulsifiers in infant nutritional products include lecithin, mono and di-glycerides, sucrose esters of fatty acids, citric acid esters of mono and di-glycerides of fatty acids, and starch sodium octenyl succinate.
Chocolates and sugar confectionery products
Emulsifiers in chocolates and sugar confectionery products provide several functions during processing and storage. In caramel, toffee, and other confectionery products that contain a disperse fat phase, emulsifiers help break down the fat into small well-dispersed fat globules.
In bubble and chewing gums, stabilizers work as plasticizers of the gum base, making the base more pliable to the mouth. They also provide water retention hydration during chewing. Common emulsifiers in gums include lecithin, glycerol monostearate, and acetylated monoglycerides. If lecithin is used, up to 1% can be used to soften chewing gums. To aid in dispersion, vegetable oil or suitable fatty emulsifiers can be added.

In chocolates, lecithin and polyglycerol polyricinoleate (E476) are the most common emulsifiers. These emulsifiers provide several beneficial effects in chocolates. However, for the most part, they provide control over flow properties in chocolates. This allows chocolates to be molded into various shapes (bars, chips, etc.).
Another emulsifier used in chocolates is sorbitan tristearate (E492). It helps delay and prevent the formation of fat bloom in chocolates. Fat bloom is discussed in more detail here. Fat bloom forms because of the appearance of fat crystals that emanate from the surface. It is characterized by the whitish coating or gray haze at the surface. Fat bloom can occur for several reasons. First, the improper processing conditions such as bad tempering and incorrect cooling method. Second, the composition of the product. And lastly, the storage condition.
Processed meats
Processed meats are meats that have been modified in order to improve its quality (taste and appearance) and shelf life stability. The processed meat segment is a huge business. In the United States alone, processed meat comprises one third of the meat industry. The processed meat segment is even bigger in Europe, where sausages are the most popular type of processed meat. Within the region, the Czechs, Germans, and Austrians consume the most sausages per capita. The basic ingredients in sausages include ground meat (pork, beef, chicken), fat, and water. An emulsifier holds these components together, forming a stable emulsion.
Citric acid esters (E472c) in bolognas, wieners, frankfurters, wieners, and hotdogs offer good dispersibility in the meat batter. Mono and di-glycerides of fatty acids also enhances the binding effects in meats. Meat batters are a type oil-in-water emulsion. The disperse phase consists of liquid or solid fat particles while the continuous phase is the water, which contains the dissolves salts and suspended proteins.
You might also like: Meat Science: Is Brown Meat Bad?
In order to ensure the uniform distribution and consistency of the emulsion, a stabilizer can be added. Common stabilizers in meat products include guar gum, cellolose gum, and locust bean gum. These stabilizers also provided additional benefits; they prevent fat migration during storage, help retain moisture, and provide viscosity control during processing.
Other references:
Chen and A. Rosenthal (2015). Modifying Food Texture. Woodhead Publishing
G. Hasenhuettl and R. Hartel (2008). Food Emulsifiers and Their Applications (2nd edition). Springer
V. Vaclavik and E. Christian (2014). Essentials of Food Science (4th edition). Springer
J. deMan, J. Finley, W. Jeffrey Hurst, Chang Yong Lee (2018). Principles of Food Chemistry (4th edition). Springer