
Studies in history have found that humans first started fermenting foods as early as 6000 B.C in the Fertile Crescent (crescent-shaped region in the Middle East). However, fermentation was a poorly understood method back then. It was until the mid-19th century when French scientist Louis Pasteur showed how fermentation works. In a series of experiments he performed, he proved that fermentation of food occurs in the presence of certain microorganisms. In 1877, through years of studies on fermentation, he published his famous book, Etudes sur la Bière (Studies on fermentation). One of the key findings in his studies is that lactic acid is produced by microorganisms (bacteria) in lactic acid fermentation. Based on Pasteur’s findings, many studies have followed, which have allowed us to have a better understanding of fermentation.
Today, fermentation can be classified into 3 types. These include lactic acid fermentation, alcoholic fermentation, and acetic fermentation. Sure, fermentation is quite a topic. So in this blog post, we’ll cover lactic fermentation, whose applications include fermented vegetables such as pickles, kimchi, sauerkraut, and fermented milks such as cheese and yogurt. Before we begin, let’s define what fermentation is.
Table of Contents
WHAT IS FERMENTATION?
Fermentation is one of the earliest and simplest forms of food preservation—no heat or artificial energy source necessary. It is a process wherein microorganisms change the sensory and functional properties of food, producing an end product that is desirable to the consumer. Basically, microorganisms do this by transforming organic substances into smaller molecules. A good example of this is alcoholic fermentation in which yeasts ferment glucose to produce carbon dioxide and alcohol.
In the case of lactic acid fermentation, lactic acid bacteria (LAB) ferment glucose to produce carbon dioxide and lactic acid. Lactic acid fermentation can be divided into two: homolactic and heterolactic fermentation.
During homolactic fermentation, one mole of glucose converts into two lactic acid moles. Up to 85% lactic acid can be produced in this reaction.
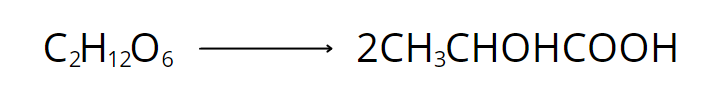
During heterolactic fermentation, on the other hand, one molecule of glucose yields one mole each of lactic acid, ethanol, and carbon dioxide. However, this reaction only produces 50% lactic acid.

What’s interesting with LABs is that they are able to produce organic acid, thereby lowering the pH or acidity level in the food. The decrease in the level of acidity creates an environment that would inhibit the growth of spoilage microorganisms and foodborne pathogens.
LACTIC ACID BACTERIA
LABs share common characteristics. They are gram-positive, catalase-negative, non-spore forming, and fermentative anaerobes. Most of their cellular energy come from the fermentation of sugars, which in return produce lactic acid. Except streptococci, LABs do not cause harm to humans. This makes them an ideal preservative agent.
How they ferment and preserve foods come from several mechanisms. The most important one is by rapidly raising the acidity level at which it inhibits the growth of undesirable microorganisms. Lactobacilli are also capable of producing hydrogen peroxide, which, too, is inhibitory to spoilage microorganisms.
Hererofermenters aside from ethanol and lactic acid also produce carbon dioxide. The following are the antifungal compounds produced by LABs during fermentation.
- Acetic acid
- Caproic acid
- Carbon dioxide
- Cyclic dipeptides
- Diacetyl
- Hydrogen peroxide
- Lactic acid
- Phenyllactic acid
- Proteinaceous compounds
- Reuterin
- 3-hydroxy fatty acids
In the food industry, LABs also serve as acidulant, and dough conditioner. In various foodstuff, LABs are added deliberately to produce many kinds of fermented foods such cereals, fish, meat, vegetables, and legumes. Interestingly, these microorganisms in foods are called probiotics, the good bacteria. They have many ways to help our body healthy, especially the digestive system.
| MICROORGANISM | FUNCTION |
| Lactobacillus | Production of yogurt, cheese, sauerkraut, pickles, beer, wine, and cider |
| Lactococcus | Fermenters of dairy products, including cheese |
| Leuconostoc | Starter culture in dairy fermentation |
| Oenococcus | Malolactic fermentation in wine |
| Pediococcus | Production of saeurkraut, cheese, and yogurt Provides butterscotch aroma to wines and beers |
| Streptococcus | Dairy manufacturing |
| Teragenococcus | Soy sauce fermentation |
| Weisella | Improving sensory properties of cheese, butter, and butter cheese |
PRODUCTS OF LACTIC ACID FERMENTATION
Common products of lactic acid fermentation are pickled vegetables such as kimchi, olives, and sauerkraut. Fermenting milk also produces products such as yogurt and cheese.
Yogurt
Yogurt is a dairy product produced by coagulating milk through lactic acid fermentation. It can be produced using whole, low-fat, or skim milk. The nonfat milk solids are raised to 12%–15% by concentrating the milk, or adding condensed milk or powdered skim milk. The milk is then pasteurized at 82°C–93°C for 30–60 minutes, and cooled at 40°C–45°C. Then, yogurt starter is added at 2% by volume and incubated for 3 to 5 hours. The length of incubation is also finished if the final product reaches a pH of 4.4-4.6 or titratable acidity of 0.85%–0.90%.
You might also like: Starter Cultures In Yogurt
The two organisms involved in yogurt fermentation is Streptococcus thermophilus and Lb. delbrueckii subsp. bulgaricus. The existence of the two in a 1:1 ratio results in lactic acid and acetaldehyde production at a greater rate than that produced by either organism when growing alone. Streptococci produce carbon dioxide, lactic acid, and formic acid. The presence of formic acid stimulates the growth of lactobacilli. Lactobacilli, on the other hand, liberate amino acids necessary for the growth of streptococci. It also leads to production of acetaldehyde, which contributes mostly to the typical yogurt flavor, and lactic acid to lower the pH to 4.4-4.6. In some yogurt culture, Lactobacillus acidophilus is present to reduce excessive aldehyde and for added health benefits.
Cheese
Cheese is a dairy product made from coagulating milk protein casein. According to the Food and Drug Administration, cheese can be coagulated with rennet, lactic acid, or other suitable enzyme or acid. For thousands of years, processing of milk into cheese makes milk readily available and less perishable. Today, fresh cheeses are also available. Cheeses like mozzarella, ricotta, and cream cheese do not require fermentation. However, many cheeses are produced by introducing starter culture bacteria to milk.
You might also like: Food Science: What Is Processed Cheese?
During cheesemaking, the addition of starter bacteria to pasteurized milk ferments it and at the same time reduces the pH level to induce curdling. To achieve curdling, two main groups of LABs can be used: moderate -temperature lactococci (such as mesophilic) and the heat-loving lactobacilli (thermophilic). Both of these groups are heterofermenters. However, the bacteria to use will depend on the particular step necessary to produce the cheese. If the milk has to be subjected to a temperature of 70°C, lactococci will effectively ferment the milk, reduce the pH to induce the formation of curd. Mesophilic bacteria can also thrive under these conditions. However, for cheeses (like hard cheeses) that require high temperature during cooking, thermophilic bacteria is ideal since they thrive in this condition. In almost all types of cheeses, an enzyme called rennet is also used to make the curd elastic and strong.
The breakdown of lactose during the fermentation process makes the cheese more digestible.
Fermented Vegetables
Perhaps vegetables are the most common applications of lactic acid fermentation. In western countries, the most commercially important ones are cabbage, cucumbers, and olives. Vegetables such as carrots, peppers, okra, onions, cauliflower, and celery are also preserved by lactic acid fermentation. Unlike other foods, these vegetables rely on the natural flora, hence fermentation may occur spontaneously. Lactic acid bacteria may thrive if the conditions are favorable to them. When fermenting vegetables, factors such as aerobic condition, temperature, moisture or water activity, and salt concentration must be taken into account. Salt concentration is particularly important.
The vegetable is submerged in a brine solution of appropriate concentration. This creates an environment unfavorable to unwanted microorganisms (spoilage microorganisms). The salt also extracts water from the vegetable, serving as a substrate for the growth of LABs. The concentration to use varies depending on the vegetable. For cabbage (sauerkraut), the brine solution should be around 2.5% and 10% for olives. Koreans use higher brine concentration when making kimchi. The brine solution can be as high as 26%. Although the standard brine solution is 15% for traditional kimchi.
You might also like: Everything You Need To Know About Kimchi
Olives receives special treatments. Prior to brining, olives are treated with lye solution for up to 8 hours to remove the bitter-tasting oleuropein.
What species of LABs are involved in vegetable fermentation?
The species involved in lactic acid fermentation depend on the stage of fermentation. For production of sauerkraut, Leuconostoc mesenteroides grows first, which produces carbon dioxide, acetic acid, and lactic acid. This is followed by the growth of Lb. brevis and then lastly Lb. plantarum. The presence of these LABs lowers the pH to below 4.0 allowing the cabbage to be stored for longer periods under anerobic conditions.
For high-salt pickles, Pediococcus cerevisiae initially grows. As the acidity lowers, the more acid-tolerant LABs Lb. plantarum and Lb. brevis thrive. Leuconostoc mesenteroides is more active in low-salt pickles, but contributes little to high-salt pickles.
In green olives fermentation, the LABS Leuconostoc mesenteroides and Pediococcus cerevisiae initially dominate. Then followed by lactobacilli Lb. plantarum and Lb. brevis.
References
M. Shafiur Rahman (2002). Handbook of Food Preservation (2nd edition). CRC Press.
Y. H. Hui, E. Ozgul Evranuz (2012). Handbook of Plant-Based Fermented Food and Beverage Technology (2nd edition). CRC Press.
M. Wallert, K. Colabroy, B. Kelly, J. Provost (2016). The Science of Cooking: Understanding the Biology and Chemistry Behind Food and Cooking. John Wiley & Sons, Inc.
V. Vaclavik and E. Christian (2014). Essentials of Food Science (4th edition). Springer.
N. A. V. Eskin (2005). Biochemistry of Foods. Academic Press.


