
When an animal is slaughtered, several physical and chemical changes occur. One of them is what we called rigor mortis. Rigor mortis (“stiffness of death”) basically refers to the stiffening of the muscle making it inextensible. Maybe you have already experienced seeing stiffness in a dead animal in a roadside accident―that is rigor mortis happening.
Rigor Mortis is the stiffening of muscle and the loss of extensibility, marking the transition from muscle to meat. This is characterized by the decline of ATP, production of lactic acid, and the ultimate pH reaching around 5.5. How rigor mortis works is a necessary thing to learn in the meat industry because it is one of the main factors that affect the overall quality of the product. Fresh meat contains interesting flavors. However, most of the savory taste commonly associated with meat develops as the postmortem physical and chemical changes of the tissue take place hours or days after slaughter.
The tenderness is particularly affected when rigor mortis sets in. Sure, immediate cutting and cooking will make a tender dish. But this is rarely practiced outside the domestic slaughtering process. Rather, the meat is allowed to undergo rigor mortis soon after death, allowing it to relax, become soft, and pliable again. Carcass that has properly gone through rigor mortis makes palatable meat. This is especially the case for handling fish, beef, and pork. Cutting or freezing the rigor-contracted muscle will only lead to large-scale tears in the muscle fiber. This will give space for water to leave, causing the tissue to become soft and mushy.
CONVERSION OF MUSCLE TO MEAT
After an animal has been killed or slaughtered, a wide range of physicochemical and biochemical reactions take place as the muscle becomes meat. This period can be divided into three phases: prerigor, rigor mortis, and post rigor state.
1. Prerigor state
This is the first phase, wherein the muscle is soft and pliable. This phase is characterized biochemically by the decrease in Adenosine 5′-triphosphate (ATP). ATP is responsible for the storing and transferring energy to make processes in cells possible. But the fall in ATP makes the muscle fibers to shorten, making the muscle to become less extendable. Creatine phosphate levels and active glycolysis also fall. Glycolysis is a series of steps that converts glucose to pyruvate or lactate, an immediate source of ATP.
The energy required for muscle contraction is generally from aerobic respiration. This makes the glucose to be broken down to yield carbon dioxide and 36 molecules of ATP. This glucose is stored as glycogen, which is broken down when necessary to supply energy.
You might also like: Myoglobin: The Protein That Dictates Meat Color
When an animal is killed, aerobic respiration and blood flow stops, and the muscle is no longer supplied with oxygen.
Postmortem glycolysis depends on several factors, including the glycogen reserve prior to harvest. Fish have small glycogen stores. When they struggle during the catch, they utilize glycogen, leaving them with a short period of time before carbohydrate metabolism ends and ATP production stops. Before slaughter, cattle are rested and fed to increase the level of glycogen.
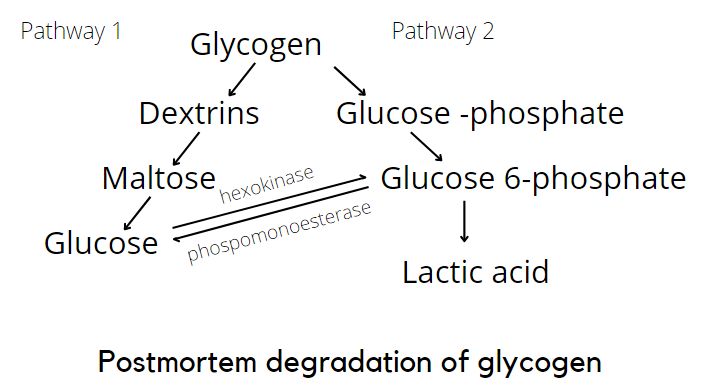
Without oxygen, cells produce less ATP from glucose. At this point, glycolysis continues, but glycogen is converted to lactic acid. The reaction continues until glycogen is depleted or the pH (acidity) of 5.5 is reached. At this pH level, denaturation of the enzymes responsible for glycolysis occurs, stopping the reaction. If glycogen stores are low, glycolysis may stop even before pH drops to 5.5, the lowest possible ultimate pH. There are instances when the ultimate pH is higher than 5.5, this is especially true if the animal is stressed or starved prior to slaughter.
2. Rigor mortis state
Synthesis of ATP via anaerobic pathway is not sufficient to achieve the required ATP. And this depletion results in the formation of actomyosin, leading to the onset of rigor mortis. Actomyosin is a protein made of up actin and myosin.
Because calcium ions cannot be pumped out of the sarcoplasm due to a lack of ATP, the active site on the thin myofilaments’ actin molecules is available to connect with the thick filaments’ myosin. From here, actin and myosin unite, to form actomyosin cross-links. In a live animal, actomyosin cross-links form and break frequently as part of contraction.
You might also like: Meat Science: Is Brown Meat Bad?
In a rigor-contracted muscle, however, this cross-link formation is not reversible since there is no available ATP anymore. The amount of actomyosin formed will determine the stiffness of the muscle. The larger the overlap of thick and thin myofilaments, the more actomyosin is formed, and the stiffer the muscle becomes.
The onset of rigor mortis is also accompanied by the decrease in water holding capacity, fresh meat’s ability to retain its own water. This is especially true when rigor mortis occurs at a high pH due to the absence of ATP and the proteins are not as close to their isoelectric point, enabling them to bind more water.
Over time, rigor declines and the muscle groups begin to extend and loosen, leading to postrigor state.
LENGTH OF RIGOR MORTIS
Although the stiffening of the muscles occurs all throughout the body, rigor mortis first occurs in the smaller muscles, and is followed by the bigger ones. In fish, rigor starts at the tail, and then the muscles harden slowly until the whole fish is stiff. The Food and Agriculture Organization discusses rigor in fish in more detail. The onset of rigor mortis occur in 1 to 12 hours postmortem, and may last a further 15-20 hours. Although several factors such as age, size, condition of the animal prior to slaughter, and external variables have to be taken into account.
| ANIMAL | DURATION OF RIGOR MORTIS |
| Fish | <1 hour |
| Poultry | 0-3 hour |
| Pork | 0-6 hours |
| Lamb | 6-10 hours |
| Beef | 6-12 hours |
For example, rigor mortis in beef completely occurs within 12 hours. But the process will take less time if postmortem temperature is lower.
Stress or physical activity prior to slaughter also affects rigor and the quality of the meat. Stress at the time of harvest influences the level of glucose and ATP in the muscle. When an animal is stressed, adrenaline is released in the body, which activates glycogen breakdown enzymes, causing the muscles to quickly enter rigor mortis. In the beef industry, this results in cuts of meat known as “dark cutters”, because of its appearance. Dark cutters form because of the change in pH of the glycogen-depleted tissue right after harvest. To prevent this, the cattle must be rested and fed before slaughter to make the rigor slow in the muscle. Doing so produces quality beef.
3. Postrigor state
At this stage, the muscles gradually tenderize, making them more organoleptically acceptable. Mammalian meat commonly achieves its optimum quality in 2 to 2 weeks following dissolution of rigor at 36°F (2°C).
The decrease in muscle is due to two reasons: enzymatic breakdown of proteins (proteolysis) and pH.
A low ultimate pH may affect its water holding capacity. But it is desirable from a microbiological point of view because it prevents microbial growth. Furthermore, during postrigor, the fall of pH level induces the protein to unravel and denature. As a result, linked proteins inside muscle fibers are lost, tenderizing the tissue.
During postmortem proteolysis and meat tenderization, three endogenous proteolytic systems are in play:
- Calpains
- Cathepsins
- Protesomes systems
Calpains are calcium-requiring proteases responsible for contracting fiber proteins. Cathepsins bind and cleave the protein backbone of muscle proteins, including collagens and connective tissues. The work of these proteins degrades the connective proteins, including actin, myosin, collagen, and trypomyosin. However, these proteases are more than just tenderizing meat.
The result of their activity also leads to the formation of new flavors. Proteases that break down entire proteins into peptides and individual amino acids take longer digestion. The amino acids they produce include aspartic acid and glutamate, which have a savory or umami flavor, while others have a sour or sweet flavor. During browning, a number of peptides and amino acids combine with carbohydrates like glucose and ribose to produce flavor molecules in cooked meat.
Many meat tenderizers in the market are protein proteases in powdered form. By soaking meat in proteases, the connective proteins are cleaved and umami flavors are formed. Proteases are often sourced from fruits like papaya or pineapple. Bromelain, a proteolytic enzyme, is the reason why stinging or burning sensation is experienced when eating pineapple.
References:
M. Wallert, K. Colabroy, B. Kelly, J. Provost (2016). The Science of Cooking: Understanding The Biology And Chemistry Behind Food And Cooking. John Wiley & Sons, Inc..
V. Vaclavik, E. Christian (2014). Essentials of Food Science (4th edition). Springer.
N. Eskin, F. Shahidi (2012). Biochemistry Of Foods (3rd edition). Academic Press.
S. Damodaran, K. Parkin (2017. Fennema’s Food Chemistry (5th edition). CRC Press.
M. Gibson (2018). Food Science And The Culinary Arts. Academic Press.
BD Sharma (1999). Meat And Meat Products Technology (Including Poultry Products Technology. Jaypee Brothers Medical Publishers (P) Ltd.



Pingback:أحلى برجر في العالم | الدحيح - الدحيح