
Invert sugar is a property of sugar formed by sucrose hydrolysis. In chemistry, hydrolysis is a process wherein one molecule of water breaks down one or more chemical bonds. In the case of sucrose hydrolysis, the action of an acid or enzyme hydrolyzes or breaks down the glycosidic bond that connects glucose and fructose as sucrose. This yields equal amounts of glucose and fructose. This process is also called inversion. And the mixture of unbroken sucrose, fructose, and glucose is invert sugar or invert syrup. Inverted sugar is valued because not only it adds sweetness in food, it also has several functional benefits.
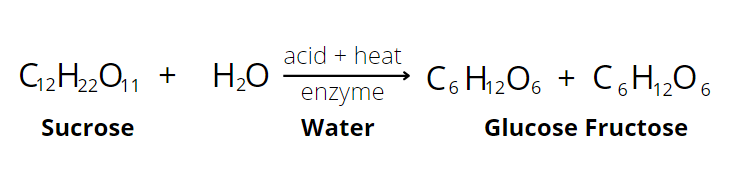
By percentage, invert sugars are 75% glucose and fructose, and 25% sucrose. Glucose that forms from inversion is less sweet than sucrose, and fructose is more sweet.
Inverted sugar is so-called because of its light-reflective properties. In food manufacturing, one device that measures % soluble sugar is a refractometer. It works using the principle of light refraction. Light slows down as it travels from air to liquid. So if light travels through a sample that contains dissolved solids (usually sugar), the light is bent or refracted. This is what gives a pencil a “bent” appearance when submerged in water. And the change of direction of the light is called refraction.
So as to why invert sugar is so-called is because its optical rotation is opposite to that of table sugar. Sucrose has a specific optical rotation of +66.5°. The hydrolysis of the glycosidic bond produces a mixture of glucose and fructose, which has a special optical rotation of -33.3°.
HOW INVERT SUGAR IS PRODUCED
Making inverted sugar is relatively easy. By heating plain sucrose and boiling it in a solution with cream of tartar or citric acid (lemon), the sugar is broken into glucose and fructose. The amount of invert sugar through acid hydrolysis depends on two factors: the amount of acid and the rate and length of heating. Too much use of acid, cream of tartar, for example, may result in excessive hydrolysis. This will produce a runny or soft sugar product. The rate and length of heating also affect inversion. Ideally, inversion should be slow and long length of heating. A rapid rate will only provide less inversion opportunity.
Further read: Food Science: The Roles of Sugar In Food
Enzyme hydrolysis can also break the dissacharide bond to produce inverted sugar. Although this method is long as the process may take several days to finish. Usually, enzyme hydrolysis is mainly achieved using invertase (also called sucrase) enzyme. Commercially available invertase is derived usually from yeast. Although plants and vegetables produce this enzyme as well to increase the availability of glucose, and fructose to make the fruit sweeter. Invertase comes in the form of powder or liquid. Its ability to break down sucrose has uses in producing candy liquid centers, fondant candies, and chocolate-covered cherries.
Now that we have defined invert sugar and how it is made, let’s talk about what it has to do with the properties of sugar.
FUNCTIONS OF INVERTED SUGAR IN FOOD
If there is regular table sugar or sucrose, why would food manufacturers use invert sugar then? Sure, table sugar comes in granules, while invert sugar comes in liquid. But it is more than that. Aside from increased sweetness, invert sugar also helps limit crystal formation, and retain moisture.
Let’s discuss further.
Sweetening products
Sucrose sweetens foods. But inverted sugar is sweeter than sucrose. When disacharide (sucrose) undergoes inversion, it yields glucose and fructose. The presence of free fructose gives invert sugar a much sweeter taste. In fact, fructose is the sweetest natural sugar. Fructose is 1.2 to 1.8 times as sweet as sucrose. For this reason, invert sugar is widely used in baking, confectionery, and pastries.
Sucrose inversion is particularly important in making jams and preserves. Making jam involves boiling added sugar and fruits together for a certain period. During boiling, two requirements are already present for sugar hydrolysis to commence: heat and acid from the fruit. These lead to inversion of half of added sucrose to glucose and fructose. The result is the increase in sugar molecules by 50%, giving a modest increase in sweetness. Furthermore, since invert sugar is more soluble in water, crystal formation is repressed. And because of this, it is easier to attain high sugar levels in jam to make it shelf life stable.
Limiting crystal formation
In many formulations, invert sugar is combined with untreated sucrose in a 1:1 ratio to control crystal formation. Here’s how invert sugar prevents crystal formation.
Invert sugar is composed of fructose, but in the form of glucose and fructose. The mixture of these sugars prevents them from crystallizing easily. In a crystal lattice, the form of the disaccharide (sucrose) and monosaccharides (glucose and fructose) do not fit together well. The result is the disaccharides staying in a viscous, syrupy state, rather than forming a crystalline solid.
Knowing how this works is specifically important for recipes of non-crystalline candies. In making sugar syrup, sugar crystals are avoided because they can crystallize the rest. In some recipes, acid sources (such as vinegar and, lemon), or sugar, such as corn syrup and glucose is added to interfere crystal formation during boiling.
Honey contains mostly glucose and fructose. Similar to inverted syrup, it can remain liquid for a long time. It also works to slow the crystalline formation. However, because of its fructose content, it readily caramelizes, which, at times, results in undesirable browning in certain preparations. If one wishes to prevent crystal formation and caramelization at the same time, acid-inverted syrups are better because of their acidity.
Many cooks favor corn syrup over sucrose because it does not caramelize readily. The varied long glucose chains in maize syrup make a tangled mess that physically obstructs the movement of both sugar and water molecules. This makes it harder for sucrose to find another crystal that it can fit onto.
Retaining moisture
Invert sugar is more hygroscopic than sucrose. Hygroscopic refers to substances that are able to pick up moisture from the air. Invert sugar is more able to do this because of the additional water molecules that can interact with two molecules of sugar (glucose and fructose) relative to one molecule of sucrose.
In general, sweeteners that are high in fructose are more hygroscopic than sucrose. These include invert sugar, molasses, and high fructose corn syrup (HFCS), This is the reason why brown sugar cookies are chewy—the invert sugar it contains attracts and retains moisture even after baking.
You might also like: What Are Humectants In Foods?
Often times, invert sugar is used together with another sweetener to achieve a certain level of sweetness while retaining other qualities. Like for example, brown sugar cookie may be chewy. But brown sugar (with invert sugar) alone will not produce a chewy sugar cookie with a glossy crystalline top. To achieve both, a combination of brown and white sugar is necessary.
References:
V. Vaclavik, E. Christian (2014). Essentials of Food Science (4th edition). Springer.
S. Damodaran, K. Parkin (2017. Fennema’s Food Chemistry (5th edition). CRC Press.
M. Wallert, K. Colabroy, B. Kelly, J. Provost (2016). The Science of Cooking: Understanding The Biology And Chemistry Behind Food And Cooking. John Wiley & Sons, Inc..
M. Gibson (2018). Food Science And The Culinary Arts. Academic Press.


