
For the most us, whenever we hear the word “ozone”, what immediately comes to mind is the ozone layer, which protects us from the harmful Ultraviolet (UV) radiation from the sun— and not the ozone (O₃) that is used in food processing. While not known to many, the use of zone in food processing is not exactly a novel technology. In fact, its history can be traced back in the late 17th century in Europe.
It was when German scientist Christian Friedrich Schönbein discovered ozone in 1839. Then a series of studies followed to prove its effectiveness against a wide range of microorganisms. In 1893, the Netherlands first used ozone full-scale for drinking water. Other European countries followed: France built the first municipal ozone plant for drinking water in 1906, and Germany employed ozone to preserve meat in 1909. America started using ozone technology in the early 20th century. In 1942, the United States started using ozone as an oxidizing agent in egg-storage rooms and in cheese-storage facilities.
In recent years, the interest in the use of ozone in food processing has grown significantly. This is due to several observable reasons. First, is the call from consumers for clean, fresh, healthy, and safe food products. Second, is that ozone is more environmentally-friendly than its counterparts. It eliminates chemical residues— the excess ozone decomposes very quickly, producing oxygen and therefore leaves no residues. And lastly, regulatory bodies,the Food and Drug Administration (FDA), in particular, view ozone as a safe component in food processing— from direct contact surfaces to fruits, vegetables, and meats
Before discussing further, let’s define what ozone is first.
Let’s dive right in.
Table of Contents
WHAT IS OZONE (O₃)?
Ozone (O₃) is a trioxygen— It is a molecule which consists of 3 molecules of oxygen bound together. Ozone occurs naturally in the Earth’s atmosphere at low concentrations of only 0.6 parts per million. Most of this is in the stratosphere at 90%. The formation of ozone in the atmosphere is continual. This happens by the action of UV radiation with atmospheric oxygen. The UV radiation breaks apart an oxygen molecule (O2). This produces 2 oxygen molecules. Since these 2 molecules are highly reactive, each combines with an oxygen molecule, forming an ozone molecule.
Ozone is a pale bluish gas. It condenses into dark blue liquid at -169.6 °F (−112 °C). And is violet-black in solid form. In most applications, particularly in food processing, its color is not noticeable. Its odor is similar to that of chlorine. Some people describe it as sweet and pungent. Van Marum first described the pungent odor of ozone in 1781. Humans can detect the odor of ozone at low concentrations of 0.02 ppm.
Ozone is partially soluble in water (0.640 ozone/L of water) and is insoluble at 140 °F (60 °C). But at standard pressure and temperature, its solubility is thirteen times more than that of oxygen. Its solubility in water increases with decreasing water temperature.
Ozone has a very short half life. Hence, when produced, will decompose very rapidly. In water, its half life is only around 30 minutes. This means that every half of an hour, its concentration decreases to half its initial concentration. For this reason, ozone must be generated on-site (a manufacturing plant for example).
PRODUCTION METHODS
Scientists measure light through a term called electromagnetic spectrum, which refers to the entire range of light that exists around us— from radio to gamma rays. Light is measured in terms of nanomemeters. Interestingly, we cannot see most of the light in the universe. According to NASA, humans can only detect wavelengths from 380-700 nm.
In the atmosphere, the sun produces the UV light that is involved in the formation of ozone in the stratosphere. Most of the UV light in the ozone layer ranges from 100-315 nm.
In commercial ozone production, the process is very similar to that. But methods vary depending on the application.
In food and beverage manufacturing, ozone is generally produced by one of two methods: By UV light, and second, by corona discharge (CD) method. In commercial scale ozone production for food manufacturing, the bulk is generated by the latter. Other methods of ozone generation methods such as radiochemical and eletrolysis are utilized to a lesser extent. These methods are either not economical or are still in development.
UV LIGHT RADIATION
But in this case, the UV ozone generator uses a light source such as low-pressure mercury UV lamps to produce narrow-band ultraviolet light. These lamps can produce light with two peaks: wavelengths of 185 and 254 nm.
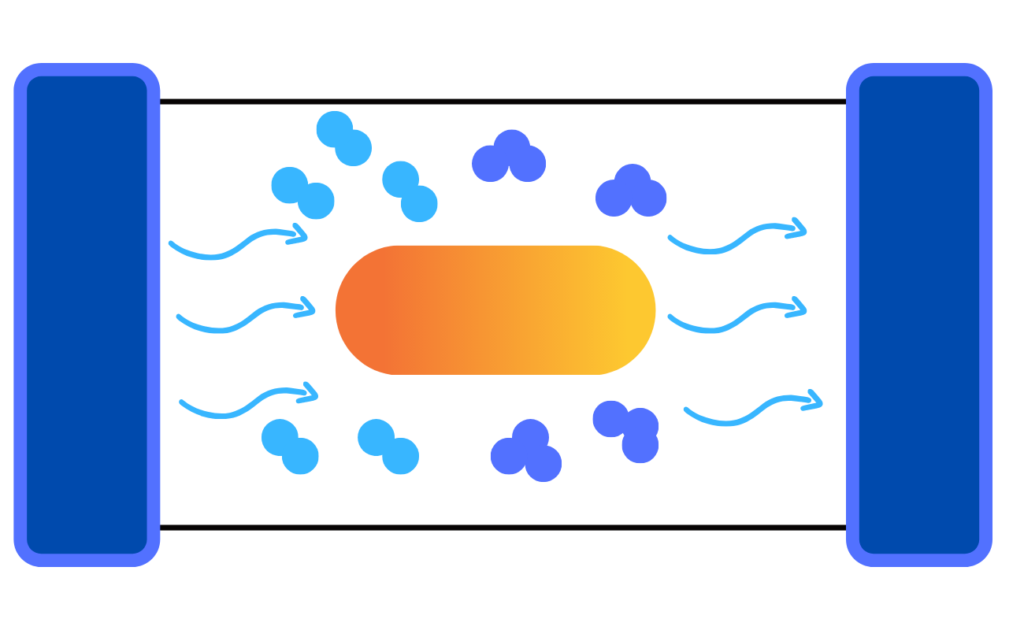
The 185 nm light is responsible for the production of ozone— the reason why it is referred to as the “ozone-producing” lamp. 185 nm is the target wavelength for commercial UV lamps in order to produce ozone. What happens here is that ambient air (or feed gas) moves through high-energy UV irradiance field that surrounds the lamp.
Then, a number of oxygen (O2) molecules split. This results in unstable oxygen radical atoms. In order to be stable, the O1 atoms attach to nearby O2 molecules, forming ozone or O3 molecules.
The 254 nm light, on the other hand, is responsible for the anti-germicidal action. Hence, not for generating a good amount of ozone. This is because at this wavelength, destruction of ozone actually occurs by dissociation. That is when UV breaks one of the oxygen bond in an ozone molecule, converting ozone back to oxygen.
A wavelength of 254 nm is optimal to destroy various harmful microorganisms—from bacteria to viruses. Furthermore, it is effective at altering the DNA, to render the microorganism unable to reproduce. In water treatment facilities, water is passed by a 254 nm lamp, which is separated from a process stream by a quartz sleeve.
For more on this, check this patent out.
CORONA DISCHARGE
For most industrial and even personal uses, the corona discharge method is the most common way of producing ozone. One main reason for this is that it can generate more ozone than UV.
Corona discharge produces ozone by introducing oxygen-containing gas through a high-voltage electrical field between two electrodes (ground electrode and dielectric medium). This electrical field, or ‘corona’ is created by diffusing an electrical charge over a dielectric surface. The dielectrics can be made of various materials— from silicon rubber to ceramics, to glass.
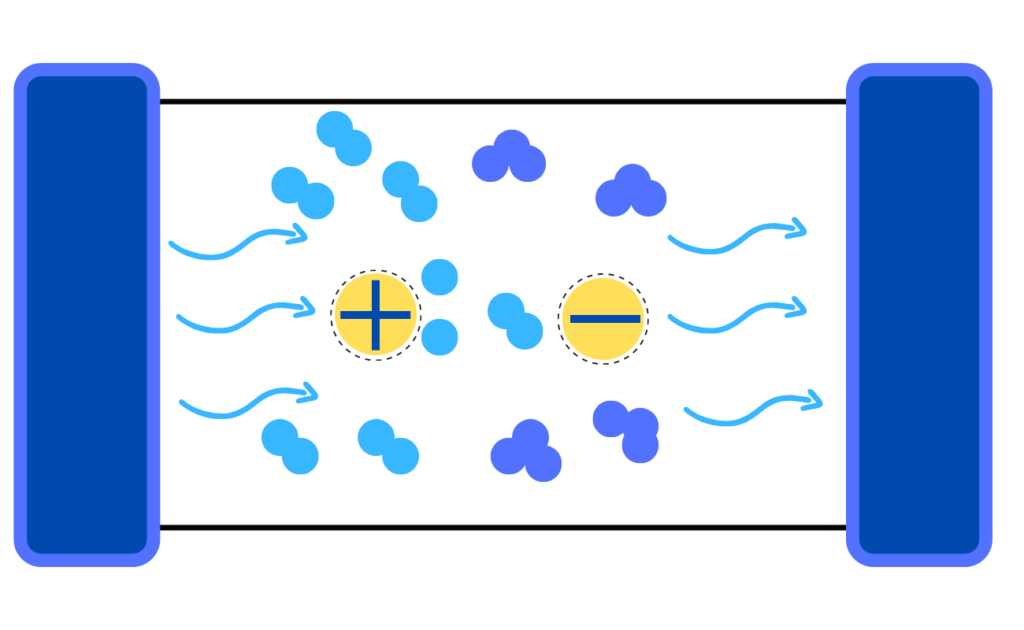
Opposite the dielectric is the reaction chamber, where the high-energy electric field is created. The reaction chamber is positioned to create a uniform space between the charged surfaces.
Similar to UV radiation, passing oxygen molecules through the electrical field splits the molecules apart into active oxygen radical atoms. Then, they readily attach to intact oxygen molecules, producing molecular ozone.
There are 3 classifications of corona discharge ozone generators according to frequency:
- Low frequency (50–100 Hz)
- medium frequency (100–1000 Hz)
- high frequency (>1000 Hz)
Corona discharge is more sophisticated than UV. Most of the supplied electrical energy fed to a CD generator produces heat. This in turn reduces the O3 generation as well as the equipment’s efficiency. For this reason, heat-removal systems are necessary. These systems use one or more of the following: water, water with freon or oil, and air.
Another drawback with corona discharge is it produces nitrogen oxide as a byproduct, which, too, may affect efficiency. One way to reduce the nitric acid formation is by removing water vapor using air dryer.
HOW OZONE WORKS
Ozone mechanism as a bactericidal
Halogens, including chlorine are effective antimicrobial agents. These halogen work by diffusion, where molecules move in or out of the cells. Ozone, on the other hand, works by oxidizing the components inside the cell wall of the microorganism. Once ozone has penetrated the cell, oxidation occurs in all the cellular components, including the DNA, RNA, proteins and the enzymes. This will eventually lead to cell lysis, or the death of the cell.
Studies have identified two major mechanisms of the destruction of target microorganisms:
- Oxidation of amino acids and sulfhydryl groups of peptides, enzymes, and proteins into smaller peptides.
- Oxidation of polyunsaturated fatty acids to acid peroxides.
The antimicrobial efficacy of ozone against the important food-related microorganisms have been studied. These studies include that on Gram-positive bacteria; including Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis, and Bacillus cereus; Gram-negative bacteria, including Pseudomonas aeruginosa and Yersinia enterocolitica; and some yeasts including Zygosaccharomyces bacilli, Candida albicans, and spores of Aspergillus niger.
What they found was that:
- Bacteria are more sensitive than fungi and yeasts against ozone.
- Gram-positive bacteria are more sensitive to ozone than Gram-negative bacteria.
- Spores are more resistant than vegetative cells against ozone.
Ozone mechanism as a virucidal agent
Researches have found out that ozone is effective in inactivating both enveloped and nonenveloped viruses in water. These studies include those on enveloped viruses, including influenza A virus, infectious bovine rhinotracheitis virus, and vesicular stomatitis viral species, and nonenveloped viruses, including infectious bovine rhinotracheitis virus, and polio type I. Ozone is also effective against bacteriophage, viruses that infect bacteria.
Studies have indicated that viruses that lack lipid envelope are more resistant to ozone oxidation that enveloped viruses.
A recent review also indicated that gaseous ozone is an effective disinfectant against novel viruses such as MERS-CoV, influenza A H1N1, SARS-CoV-1 or SARS-CoV-2 (COVID-19).
FOOD APPLICATIONS
Ozone is a very popular alternative to traditional disinfectants, particularly chlorine. One clear reason for this is chlorine’s low inactivation rate, as limited by regulations. Other common disinfectants, including sodium hypochlorite, calcium hypochlorite, hydrogen peroxide, and peroxyacetic acid are also not very effective against specific microorganisms.
Like chlorine, ozone is a powerful oxidant, but is 1.5 times more potent. In food processing, ozone is typically used at a concentration of 0.1–0.5 ppm. This is strong enough to kill many microorganisms— from Gram-positive and Gram-negative bacteria, to viruses and protozoa.
Fruits and vegetables
Further reading: Sanitizing Fresh Fruits and Vegetables Using Chlorine
During post-harvest of fruits and vegetables, producers use ozone to remove pesticide and chemical residues, inactivate pathogens and spoilage microorganisms, and extend the shelf life.
Application ozone in produce can be done by gaseous treatment or ozone dissolved in water. In fresh-cut vegetables, ozone can be added to water for sanitation. This reduces the microbial load in the product and extends the shelf life. In blackberries and grapes, ozonation can reduce the fungal decomposition.
One study found that apples treated with ozone resulted in reduced weight loss and extended shelf life. The increased shelf life of fruits is attributed to the fact that it can chemically affect ethylene, the gas responsible for ripening in fruits.
You might also like: Are There Fruits That Continue To Ripen After Harvest?
Other studies have also proven that aqueous ozone treatment is effective in fruits and vegetables, including black pepper, broccoli,lettuce, carrots, tomatoes, and grapes.
Putting up an ozone generator system may be costly at first, but implementing this system has been proven to be economical. Producers who used ozone were able to increase their yield enough to recover their investment during the first season of implementation. For example, according to this review, treating produce such as apples and salad mixes with ozone allows for less frequent change of flume water. This also reduces the maintenance and wastewater disposal cost. An additional benefit of using ozone is the reduced weight loss of produce during storage.
Fish and seafood
Fish is highly perishable. The main reason for this is their biological composition. Once fish die, the bacteria quickly consume them. In return, they convert the proteins into amino acids. And in return, foul-odor compounds such as hydrogen sulfide are produced.
Further read: These 5 Signs Will Tell You If Fish Is Fresh
To slow down quality degradation, seafoods can be sprayed with or soaked in ozonated water. But efficacy may differ. In one case, soaking mechanically-peeled shrimp in ozonated water was more effective than spraying. Longer treatment and higher ozone concentration also were more effective at reducing spoilage bacteria in shrimp.

In washing fish and fish fillets, dipping the product in ozonized water was proven to be effective in reducing the number of microbiological flora. Results also showed that it had little effect in the quality of the product. Biochemical analyses confirmed that using ozone did not negatively affect the quality of fish. And instead inhibited the mechanisms involved in oxidation and lipid hydrolysis.
Another study applied short ozone at 6 ppm as a live pre-treatment of tilapias before storing at a temperature of 32 °F (0 °C) and 41 °F (5 °C). Results revealed that ozone pre-treatment extended their shelf life by 12 days— a 40% increase. And it also increased the quality characteristics during 1 month of storing. Based on these results, the authors concluded that the combination of ozone during pre-treatment and storage temperature of 32 °F a feasible way of extending the shelf life of fish, thus extending marketability and exportation potential.
Meat products
Several studies have revealed how effective ozone is in surface decontamination of meat.
In a 2003 study by Castillo et al, the results showed that ozone spray treatment can be as effective as that of water spray treatment in beef carcass surface decontamination. However, using ozone allowed for lower pressure while achieving similar decontamination effect. In water spray treatment, the pressure used was gradually increased up to 400 lb/in2. Whereas as the ozone treatment only needed up to 80 lb/in2 of spray pressure. The beef carcasses involved in the study were inoculated with feces, which contained E. coli 0157:H7 and Salmonella typhimurium.
In 1989, a study revealed that beef carcasses under ozone atmosphere can retard the growth of psychrotrophic bacteria, or cold-loving bacteria. The authors did this by placing one beef carcass paired side into a cooler with continuous ozone generation.
In 2004, a study by Novak and Yuan evaluated the impact of ozone treatment combined with mild heat pretreatment on C. perfringens spores. The beef samples involved in the study were subsequently packed under Modified Atmosphere Packaging (MAP). Results showed that the ozone treatment made the C. perfringens spores to remain dormant for up to 10 days. The effect on spore germination intensified with increasing ozone ozone concentration.
In poultry meat, a 1979 study by Yang and Chen evaluated the effects of ozone on poultry meat (thigh and breast) microflora. To produce an inoculum, Natural poultry microflora were incubated. Then, the inoculated poultry meat was washed with 3.88 mg/L of ozone and a flow of 2050 mL/min for 20 minutes. Results revealed that ozone washing, combined with refrigerated storage, reduced microbial counts. It was specifically effective at reducing Gram-negative rods. Furthermore, the ozone treatment extended the shelf life of the poultry meat by 2.4 days.
Bottled water
Since 1906, ozone has been utilized commercially to treat drinking water or bottled water. In 1982, the FDA approves the Generally Recognize As Safe (GRAS) status of ozone for bottled water disinfection.
Ozone is introduced to water just right before filling. A common way to achieve this is by injecting ozone into a large tank or container of water until the target concentration is attained. The water is then transferred into individual bottles. The ozone concentration in bottle water must be carefully checked. Low concentration may permit the growth of microorganisms. But if the ozone concentration is too high, the bottle may develop undesirable aftertaste. The International Bottled Water Association recommends 1.0 to 2.0 milligram per liter (mg/L) of ozone with a contact time of 4 to 10 minutes to ensure proper disinfection.
METHODS OF MEASURING OZONE

In food processing, ozone content is measured to determine if the concentration, which is usually measured in mg/L, is sufficient for disinfection. Similar to chlorination, this concentration is multiplied by contact time (minutes) of ozone to determine the Ct value, expressed in mg/min/L. The Ct value is the product of the concentration of ozone and the contact time with the water being disinfected. Studies revealed that a Ct value of 0.48 mg/min/L can achieve a 99.9% or 3 log reduction of microbial populations in most food processing setting.
The methods of measuring ozone content in food processing are categorized into two: colorimetric analysis and instrumental (electronic).
Colorimetric analysis
Like the name suggests, colorimetric analysis utilizes a color reagent to determine the concentration of ozone. There are 3 main colorimetric methods: iodometric titration, N, N-diethyl-p-phenylenediamine or (DPD), and indigo trisulfonate.
Iodometric titration. In this method, ozone reactions with potassium iodide (KI), forming iodine (I2). Then, titration of iodine with thiosulfate (Na2S2O3) to a starch indicator follows to determine the concentration of ozone.
DPD. In this method, potassium iodide is added prior to analysis. Then ozone reacts with iodide to release iodine, reacts with DPD, producing pink color. The stronger the intensity of the pink color, the stronger the concentration of ozone.
Indigo trisulfonate. With indigo trisulfonate, the method is quantitative and simple. indigo trisulfonate readily reacts with ozone. Bleaching the blue indigo color is in direct proportion to the concentration of ozone. Generally, the sample is adjusted to a pH of 2 to minimize the destruction of ozone. And to prevent interference with chlorine, which decolorizes ozone at a moderate rate, malonic acid is included in the formulation.
Instrumental
Instrumental methods include oxidation/reduction potential, membrane probe, and UV absorbance. These methods allow testing of ozone in real-time and prevent ozone degassing during testing.
Oxidation/reduction potential (ORP). ORP is the substance’s ability to gain or lose an electron to an electrode, and therefore be reduced or oxidised. When water is ozonized, ozone will increase the oxidation capacity of the water or the ORP. The OPR is measured using a probe or a voltmeter. What the voltmeter measured is then converted to pH or the oxidation/reduction potential.
Membrane probe. This method of measuring ozone is similar to ORP. What is unique with membrane probe is the gas permeable membrane over the platinum electrode. In order to measure the concentration of ozone, ozone has to diffuse through the membrane to reach the electrode, which then generates the voltage.
UV absorbance. Remote measurements of ozone depend mostly on its unique absorption of UV radiation. In water, ozone has an absorbance peak in water of around 258 nm. While UV absorbance is commonly employed in gas analysis, it is also used for measuring ozone concentration of water free of UV-absorbing impurities.
REGULATIONS
In the US, ozone is approved for use in various applications. These include surface decontamination, process water disinfection, process equipment sanitation and food storage areas, to name a few. In 1982, the U.S. Food and Drug Administration granted ozone a Generally Recognized as Safe (GRAS) status for disinfection of bottled water. The FDA also accepts ozone as an antimicrobial agent when used as a gas or dissolved in water.
The US Department of Agriculture approves the use of ozone on all meat and poultry products in accordance with current industry standards of good manufacturing practice.
Other references:
J. Jay, M. Loessner, and D. Golden (2005). Modern Food Microbiology (7th Edition). Springer publishing
C, O’Donnell, B. Tiwari, P. Cullen, and R. Rice (2012). Ozone in Food Processing. Blackwell Publishing Ltd.


