Water activity (aw) is one of the few important parameters that dictate the shelf life stability of food—a food safety concern. But before we get to that, let’s define first what water activity is. More often, students think water activity and moisture content are the same thing. Well, they are not. Moisture content refers to the amount of water in the food. While water activity is the amount of unbound or free water. Hence, water activity is the available water for many chemical, physical, enzymatic, and more specifically microbiological reactions.
Another definition of water activity is the ratio of the amount of vapor pressure exerted by food (Ps) to the saturated vapor pressure of pure water at the same temperature (Pw).
Aw = PS / PW
Yes, food does exert vapor pressure. The amount of pressure depends on the water available, the food composition and temperature. For example, sugars and salts are more capable of lowering the vapor pressure than proteins and starch (larger molecules.) It is why sugars (jams) and salts (dried fish) are effective antimicrobial agents.
You might also like: 5 Main Functions of Salt (Sodium Chloride) In Food
The values of water activity range from 0 to 1.0. Pure water has a water activity of 1.0, all is readily available for use. Perishable food products such as fresh vegetables, fruits, and meats have a water activity of 0.98 to 1.00. And the water activity of dried or dehydrated foods ranges between 0.60 to 0.66.
See below for the list of water activity values of some common foods.
WATER ACTIVITY VALUES
| FOOD | TYPICAL WATER ACTIVITY (aw) |
| Fresh meat | 0.98 |
| Pasta | 0.50 |
| Honey | 0.50-0.65 |
| Dried fruits | 0.60-0.65 |
| Fresh vegetables | 0.97-0.99 |
| Dried meat | 0.80-0.92 |
| Marmalade, jams, and jellies | 0.75-0.80 |
| Fresh fruits | 0.97-0.99 |
| Most cheese varieties | 0.93-0.96 |
| Raisins | 0.51-0.56 |
| Wheat flour | 0.72 |
| Milk powder | 0.20 |
| Instant coffee | 0.20 |
| Potato crisp | 0.08 |
| Biscuits | 0.20 |
| Soy sauce | 0.80 |
MICROORGANISM REQUIREMENT
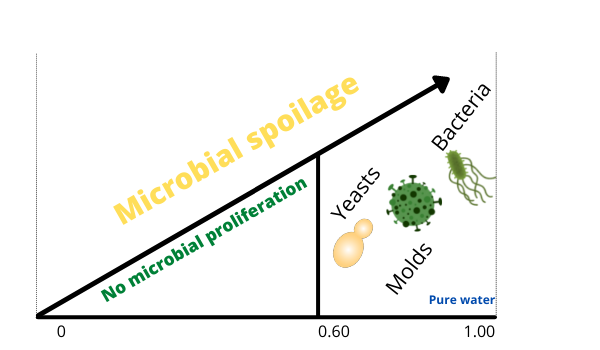
The water activity requirement for microorganisms to thrive vary as well. While some types of microorganisms require an environment with high amount of unbound water, others can survive in an environment of low water activity.
Generally, bacteria require a higher water activity than most yeasts and molds. They are the main reason of spoilage in fresh vegetables and meat. Most of these, particularly spoilage bacteria, grow at above 0.90 aw. Pathogenic bacteria, the ones that cause foodborne illnesses, do now grow well below 0.93 aw. The specie Staphylococcus aureus is a notable exception, which can grow at 0.86 aw.
You might also like: Factors That Affect Growth of Harmful Microorganisms
To inhibit the growth of bacteria, the water activity should be least 0.90 or below. Although a water activity at this level still permits the growth of fungi. Generally, molds and years stop growing at a water activity of around 0.85 or below. However, most of these fungi do not produce toxins at mentioned water activity levels.
Food items that have a water activity of 0.60 or lower do not permit the growth of most microorganisms. One good example of food that is highly shelf life stable is honey. The water molecules contained in the honey are hydrogen-bonded to the high amount of sugar (fructose for example) molecules. This therefore means no free water available for microorganisms to grow. Other foods with low water activity include dried fruits, nuts, potato crisp (chips), ground coffee beans, and pasta. They are safe indefinitely as long as kept properly; exposing them to moisture may promote mold growth.
See below for the list of water activity requirements of important microorganisms in food.
List of minimum water activity for some species of microorganisms
| MICROORGANISM TYPE | SPECIE | MINIMUM WATER ACTIVITY |
| Bacteria | Acinobacter spp | 0.95-0.98 |
| Bacteria | Bacillus cereus | 0.92-0.95 |
| Bacteria | Clostridium botulinum | 0.90-0.98 |
| Bacteria | Clostridium perfringens | 0.93-0.97 |
| Bacteria | Escherichia coli | 0.94-0.97 |
| Bacteria | Lactobacillus spp | 0.90-0.96 |
| Bacteria | Listeria monocytogenes | 0.92 |
| Bacteria | Salmonella spp | 0.93-0.96 |
| Bacteria | Staphylococcus aureus | 0.83-0.92 |
| Most spoilage bacteria | 0.90-0.91 | |
| Mold | Aspergillus spp | 0.68-0.90 |
| Mold | Aspergillus flavus | 0.78-0.90 |
| Mold | Aspergillus niger | 0.80-0.84 |
| Mold | Botrytis cinerea | 0.93 |
| Mold | Fusarium spp | 0.82-0.92 |
| Mold | Mucor spp | 0.80-0.93 |
| Mold | Penicillium spp | 0.78-0.93 |
| Most molds | 0.85-0.98 | |
| Yeast | Saccharomyces bailii | 0.80 |
| Yeast | Saccharomyces cerevisiae | 0.90-0.94 |
| Yeast | Saccharomyces rouxii | 0.62 |
| Osmophilic yeasts | 0.60-0.78 | |
| Spoilage yeasts | 0.88 |
WAYS OF LOWERING THE WATER ACTIVITY IN FOOD
Like we already know, food products have varying water activity. Generally, lowered water activity below the optimum increases the lag phase of growth and decreases the growth rate as well as the size of the microbial population. Learn more about the bacteria growth curve.
To lower their water activity and lengthen their shelf life, the preservation methods, including dehydration, solute addition, and crystallization are commonly employed. Normally, two or more methods are used for best effect—salting and drying of fish, for example. Dried and dehydrated foods are very shelf life stable and remain safe for consumption up to 1 year even without refrigeration.
Osmotic dehydration
Foods that commonly contain solutes such as salt and sugar have a low water activity. Salt and sugar decrease the available water in the food through a process called osmosis. By definition, osmosis is a process where solvent (water) molecules pass through a semipermeable membrane from a solution of higher solute (salt or sugar) concentration to a solution of lower solute concentration, equalizing both sides of the membrane. The difference in the osmotic pressure and the binding of free water result in killing the microorganisms. Examples of foods preserved using salt or sugar include jams, fruit preserves, corned beef, bacon, and beef jerky.
Drying

Drying, one of the oldest forms of food preservation physically removes water from food by evaporation. It effectively inhibits the growth of bacteria, yeasts, and molds by lowering the water activity to 0.60 or lower. During the ancient times, our ancestors only used the high heat of the sun to dehydrate, decrease the moisture content, and lower the water activity of their food to extend the shelf life. Today, there are many types of food drying methods, which are faster and more convenient to execute. These methods include freeze drying, convection drying, infrared radiation drying, by using a food dehydrator or oven, to name a few. Common dried foods include vegetables, fruits, meat, and fish.
Freezing
In freezing, the conversion of water into solid state influences water activity since ice crystals render water unavailable for reactions. Thus, the food is preserved and its its shelf life is extended. And because water is in a frozen state, determining the water activity of food is not necessary.
WATER ACTIVITY AND FOOD SAFETY IN FOOD MANUFACTURING
In the food manufacturing setting, the water activity is one of the many product characteristics that are controlled to keep the hazards in check using the internationally recognized food safety management system called Hazard analysis and critical control points (HACCP). The water activity in the food product is periodically determined by obtaining a sample from the production area to the food laboratory for testing using a water activity meter. There are 3 kinds of instruments for determining the water activity in food: dewpoint hygrometers (chilled-mirror), resistive electrolytic hydrometers, and capacitance sensors.
The mechanic of how they work is simple. A food sample is placed enclosed in a chamber of the equipment. With the container sealed, the liquid-phase water in the sample equilibrates with the relative humidity or vapor-phase water in the headspace. With the two at equilibrium, relative humidity of the headspace can be determined along with the water activity, since it is basically the ratio of vapor pressure exerted by food to the saturated vapor pressure of pure water at the same temperature.
Water activity, and other parameters such as pH, gas atmosphere, and the temperature are product criteria that are used as a quick measure of judging the safety of of particular foods. For example, acid foods, generally must have a pH of 4.6 and a water activity of equal to or greater than 0.85. Aside from preservative treatments, water activity can also be controlled by applying additive called humectants. Humectants such as sugar, salt, honey, and glycerine lower the water activity by binding water and depressing the vapor pressure.
Aside from microbial spoilage, a product with controlled water activity also prevents unfavorable changes such browning, oxidation, and change in taste.
Other references:
P. Golob, G. Farrell, and J. Orchard (2002). Crop Post‐Harvest: Science and Technology: Principles and Practice (Vol. 1). Blackwell Publishing
V. Vaclavik, and E. Christian (2014). Essentials of Food Science (4th ed.). Springer-Verlag New York
J. J. Provost, K. L. Colabroy, B. S. Kelly, M. A. Wallert (2016). The Science of Cooking. John Wiley and Sons, Inc.
P. Fellows (2000) Food Processing Technology (2nd Ed.) Woodhead Publishing