
Have you ever been curious about the nature of acrylamide and how it develops in the food we regularly consume? Acrylamide is a naturally occurring compound that emerges when specific foods undergo high-temperature cooking methods like frying, baking, or roasting. The concern surrounding acrylamide stems from its potential impact on our health, particularly its association with cancer risk.
The purpose of this blog is to unravel the chemistry behind acrylamide, its formation process, and the consequences it may have on human well-being. Furthermore, we will explore the latest scientific research on the potential health hazards linked to consuming acrylamide.
You might also like: Korean Study: Overcooking With Air Fryers Creates Toxic
By gaining insight into the chemistry and formation of acrylamide, we can better understand its impact on the human body. We will address common questions regarding the effects of acrylamide and explore strategies for reducing its presence in our diets.
WHAT IS ACRYLAMIDE?
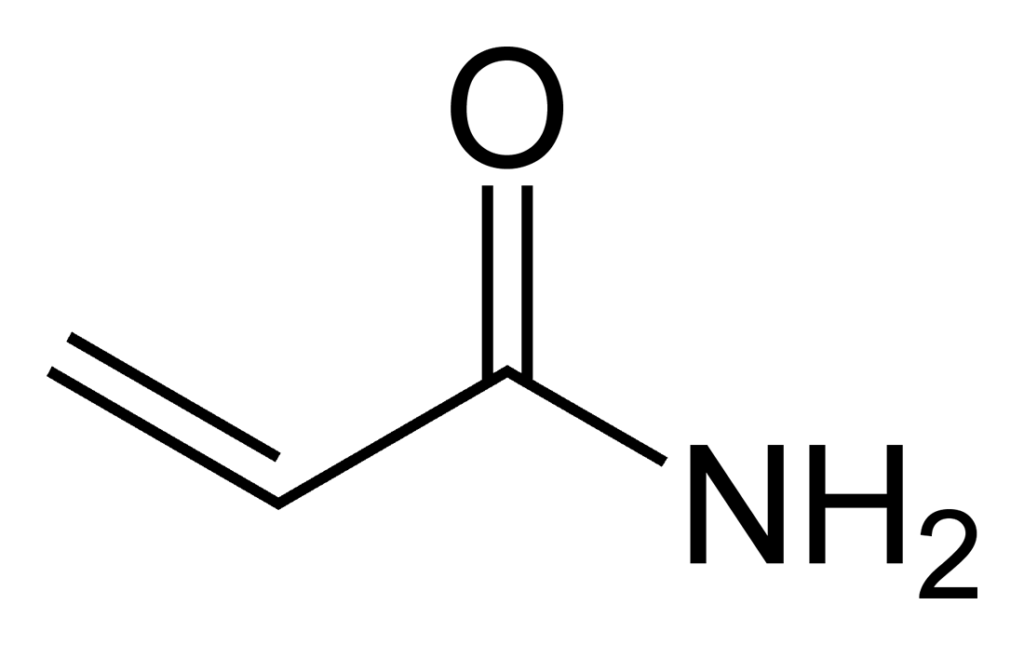
Acrylamide forms through a chemical reaction known as the Maillard reaction. This reaction occurs when certain amino acids and sugars in food react at high temperatures, typically above 248°F (120°C).
Acrylamide forms during frying, broiling, baking, and roasting due to the high temperatures involved, which promote the Maillard reaction and acrylamide formation.
These cooking methods also create a dry heat environment that allows for water evaporation and concentration of sugars and amino acids, further facilitating acrylamide formation.
Boiling and steaming, with their lower temperatures and presence of liquid water, are less conducive to acrylamide formation due to the absence of dry heat and the dilution of sugars and amino acids. In facts, conducted studies did not detect acrylamide in unheated and boiled foods.
Acrylamide, when present in high concentrations, is acknowledged as a neurotoxin. Animal studies conducted with acrylamide concentrations thousands of times higher than those typically found in food did not show an increased risk of cancer, although the applicability of these findings to humans remains uncertain. Ongoing research is exploring the potential connection between acrylamide and certain types of cancer, suggesting a possible increased risk. Despite the inclination to minimize acrylamide intake, a preliminary study revealed its presence in 40% of the American diet.
Mitigating acrylamide consumption can present difficulties due to its formation during everyday cooking practices. Nevertheless, there are approaches that can aid in reducing its presence in our diets. These strategies encompass refraining from overcooking or charring foods, selecting cooking techniques that generate lower levels of acrylamide (such as steaming or boiling), and embracing a diverse diet that emphasizes fruits, vegetables, and whole grains.
THE CHEMISTRY BEHIND ACRYLAMIDE FORMATION
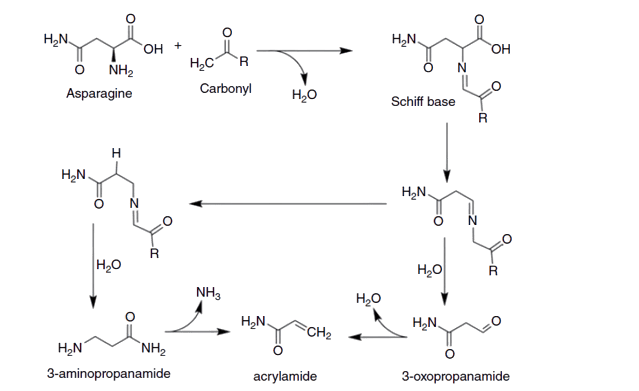
Acrylamide formation requires specific compounds to be present in the food during high-temperature cooking. The main compounds involved in the formation of acrylamide are sugars (particularly glucose and fructose) and the amino acid asparagine. Here’s a breakdown of the compounds required for acrylamide formation:
- Sugars: Sugars are essential for the Maillard reaction, which is responsible for acrylamide formation. During high-temperature cooking, the sugars undergo a series of complex chemical reactions with other compounds, including amino acids, resulting in the browning, aroma, and flavor development in cooked foods.
- Asparagine: Asparagine is an amino acid naturally present in many foods, particularly those rich in protein, such as potatoes, grains, and coffee beans. When combined with sugars during cooking, asparagine plays a crucial role in the formation of acrylamide. Under high heat conditions, the Maillard reaction occurs between asparagine and reducing sugars, leading to the production of acrylamide.
While sugars and asparagine are key components for acrylamide formation, it’s worth noting that not all foods that contain these compounds will necessarily produce significant amounts of acrylamide.
It’s important to note that the exact mechanisms and interactions involved in acrylamide formation are complex and not yet fully understood. Studies have shown that reducing sugars containing a free aldehyde group can react with asparagine at temperatures exceeding 212°F (100°C), resulting in the formation of an N-glycoside compound. This N-glycoside is subsequently cleaved at the C-N bond, leading to the production of an intermediate that ultimately yields acrylamide. A study conducted in 2003 proposed a pathway illustrating the transformation of N-glycoside into acrylamide. Moreover, it has been observed that substances such as 2-deoxyglucose, glyoxal, and glycerol can also combine with asparagine to synthesize acrylamide.
FOODS COMMONLY ASSOCIATED WITH ACRYLAMIDE
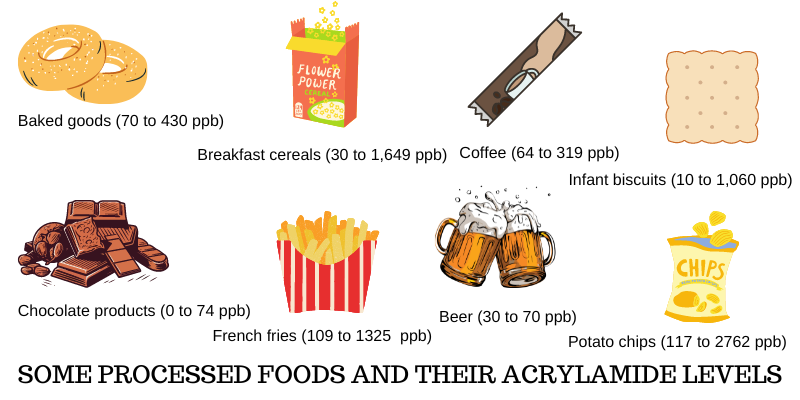
Acrylamide is found in a range of foods that undergo high-temperature cooking processes. Fried potato chips (16-30%), potato crisps (6-46%), coffee (13-39%), pastry and sweet biscuits (10-20%), bread and crisp bread (10-30%) are the main contributors to the dietary exposure of western populations to acrylamide. Other foods contribute less than 10%.
The proportion of each food item in the total intake of acrylamide varies depending on the composition of the food basket in different countries. For instance, in Sweden, coffee contributes 39% to the total exposure while in the Netherlands it is only 13%. In the United States, fried potato products account for 35% of exposure while coffee accounts for only 7%.
Here are the food items commonly associated with acrylamide formation:
Potatoes
When potatoes are cooked at high temperatures, such as frying or roasting, the naturally occurring sugars and the amino acid asparagine present in the potatoes undergo a chemical reaction known as the Maillard reaction. This reaction leads to the formation of acrylamide, resulting in the characteristic golden-brown color and crispy texture of potato products like French fries and potato chips.
Coffee
Acrylamide is naturally formed during the roasting of coffee beans. The high temperatures involved in the roasting process cause the Maillard reaction to occur, resulting in the formation of acrylamide. The amount of acrylamide in coffee is primarily determined by the duration and temperature of the roasting process. On average, coffee contains between 249 and 253 μg of acrylamide. A study indicated that coffee substitutes have the highest level of acrylamide at 818 μg/kg, followed by instant coffee at 358 μg/kg, and then roasted coffee at 179 μg/kg. I have discussed acrylamide in coffee in a separate post.
Baked Goods
Baked goods, such as cookies, crackers, bread, pastries, and cakes, contain ingredients like flour, sugar, and fats, which are prone to acrylamide formation when exposed to high heat during baking. The Maillard reaction between the sugars and amino acids in these ingredients leads to the production of acrylamide, contributing to the desirable texture and flavor of baked goods.
Snack Foods
Snack foods like pretzels, corn chips, and popcorn are often processed at high temperatures, making them susceptible to acrylamide formation. The combination of starches, sugars, and high-temperature cooking methods during snack food production can lead to the formation of acrylamide.
Potato chips, being the most popular among consumers, often exhibit elevated levels of acrylamide in comparison to other snacks. This disparity can be attributed primarily to the naturally higher concentrations of reducing sugars and asparagine amino acid present in potatoes.
In contrast, vegetable chips and tortilla chips generally contain lower amounts of acrylamide when compared to potato chips. This difference is primarily due to variations in their composition and cooking methods. A study revealed that the levels of acrylamide in potato chips ranged from 117 to 2762 parts per billion (ppb), whereas tortilla chips demonstrated acrylamide levels ranging from 130 to 196 ppb.
Breakfast Cereals
Certain breakfast cereals, especially those made from grains like oats or rice, can contain acrylamide. This is because these cereals often undergo processes such as toasting or extrusion at high temperatures, which can trigger the formation of acrylamide through the Maillard reaction.
HEALTH RISKS
The discovery of acrylamide as a neurotoxin and carcinogen in heated foods has raised concerns about its potential health effects. When ingested, acrylamide is metabolized in the body and can form reactive compounds that may bind to DNA and proteins. This can potentially lead to genetic mutations and cellular damage.
Research has indicated that the consumption of foods high in acrylamide is associated with a higher incidence of certain cancers in humans, including ovarian, endometrial, breast, and kidney cancers. This was confirmed in several studies presented. In a 2010 study conducted by Harvard School of Public Health (HSPH), it revealed a heightened risk of ovarian and endometrial cancer in non-smoking post-menopausal women who regularly consume food and beverages with elevated acrylamide levels.
However, our current knowledge about the comprehensive effects of acrylamide on human health is limited. The available evidence primarily stems from studies conducted on laboratory animals rather than direct investigations into human exposure to acrylamide from food sources. Various organizations, including the US Food and Drug Administration (FDA), European Food Safety Authority (EFSA), and the American Cancer Society acknowledge the necessity for further research to fully comprehend the complete impact of acrylamide on human health.
To date, evaluations of epidemiological studies conducted on diverse populations suggest that there is minimal evidence linking dietary acrylamide to the risk of developing most common types of cancer. However, ongoing research endeavors will provide further insights into the potential correlation between acrylamide levels in foods and an increased risk of cancer.
REDUCING ACRYLAMIDE IN YOUR DIET
Although it is challenging to completely eliminate acrylamide from the diet, there are several measures you can take to reduce its intake. Within the United States, the FDA governs the permissible levels of residual acrylamide in materials that come into contact with food. However, there are presently no specific regulations concerning the presence of acrylamide in food products themselves. In 2016, the FDA released guidelines aimed at assisting the food industry in minimizing acrylamide content in select foods. It’s important to note that these guidelines serve as recommendations rather than enforceable regulations.
At home, you can follow simple steps that can effectively lower your consumption of acrylamide:
Avoid Overcooking or Burning Foods
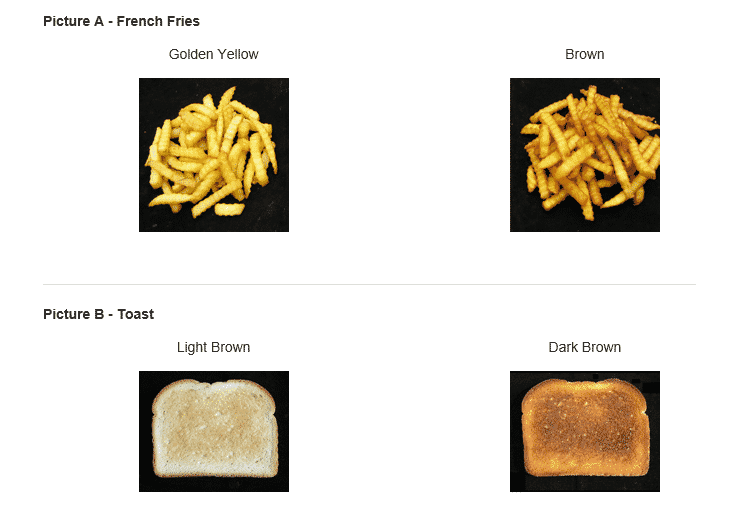
Acrylamide formation is more likely to occur when foods are overcooked or burned. The darker the food is, the more arcylamide has formed. To minimize acrylamide levels, be mindful of cooking times and temperatures. Cook food only until it turned golden yellow or light brown. (See above illustration as provided by the FDA). Avoid excessive browning or charring foods, as this can increase acrylamide formation. Or better yet, opt for cooking methods that retain moisture, such as steaming or boiling, which tend to produce lower levels of acrylamide.
Opt for Cooking Methods with Lower Acrylamide Production
Certain cooking methods are known to generate less acrylamide compared to others. Steaming, boiling, and microwaving are gentler techniques that can help reduce acrylamide formation. When applicable, choose these methods over frying, baking, or roasting at high temperatures.
For instance, when preparing potatoes, steaming or boiling them instead of frying or baking at high temperatures can significantly reduce acrylamide formation. By opting for gentler cooking methods like steaming or boiling, you can mitigate the risk of excessive acrylamide production while still enjoying delicious and nutritious dishes.
Embrace a Varied Diet
You can reduce your acrylamide exposure by include a variety of foods in your diet. Make sure to include plenty of fruits, veggies, and whole grains in your diet. These foods contain lower levels of acrylamide and have several nutritional benefits..
Instead of relying primarily on processed snacks like potato chips or French fries, include a variety of fruits, vegetables, and complete grains in your meals.
Storage and Preparation
Proper storage and preparation methods can also play a role in reducing acrylamide. Store potatoes and other starch-rich foods in a cool, dark place instead of the refrigerator. The asparagine content of potatoes and similar foods is not significantly affected by storage conditions. However, it is known that long-term storage of potatoes below about 39°F (4°C) increases the level of reducing sugars, which potentially increases acrylamide formation during cooking.
Additionally, soaking raw potato slices in water for 15-30 minutes before frying can help remove some of the starch and lower acrylamide levels. Starch is a precursor to acrylamide formation during cooking. By soaking the potatoes in water for 15-30 minutes prior to frying, some of the starch on the surface of the potato slices can be leached out.
The water acts as a medium for drawing out the excess starch, which may contribute to a reduction in acrylamide formation during the cooking process. It is important to note that this technique may not completely eliminate acrylamide, but it can be a helpful step in minimizing its levels. I have discussed this in a separate post further: Why Soaking Potatoes In Water Is Important.
References:
J. Provost, K. Colabroy, B. Kelly, M. Wallert (2016). The Science of Cooking: Understanding the Biology and Chemistry Behind Food and Cooking. John Wiley & Sons, Inc.
N. A. Michael Eskin, F. Shahidi (2013). Biochemistry of Foods (3rd edition). Academic Press.
P. Cheung, B. Mehta (2015). Handbook of Food Chemistry. Springer.
A. Zeb (2019). Food Frying: Chemistry, Biochemistry, and Safety.John Wiley & Sons Ltd.
S. Damodaran, K. Parkin (2017). Fennema’s Food Chemistry (5th edition). CRC Press.
H. Belitz, W. Grosch, P. Schieberle (2009). Food Chemistry (4th Edition). Springer.
J. Velisek (2014). The Chemistry of Food. John Wiley & Sons Ltd.


